What is the Debye-Huckel-Onsager’s equation?
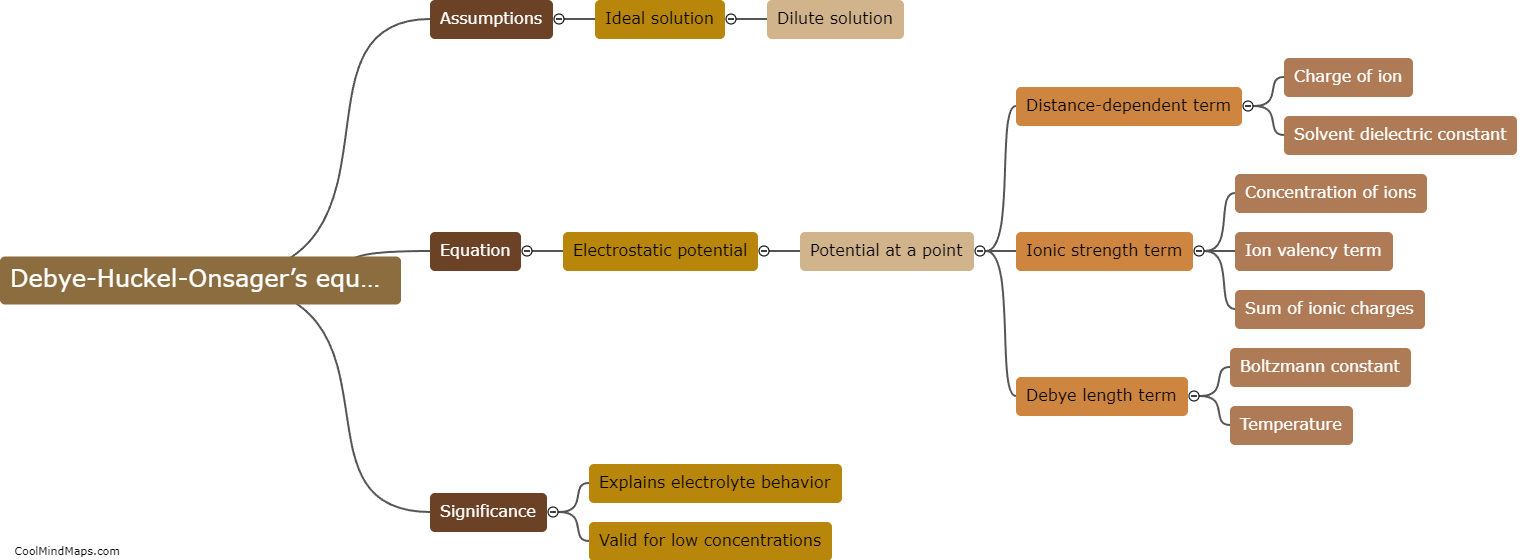
The Debye-Huckel-Onsager's equation is a mathematical expression that describes the behavior of electrolyte solutions. It was formulated by Peter Debye, Erich Huckel, and Lars Onsager in the early 20th century. This equation explains the effect of ionic strength on the activities of ions in solution. It takes into account the mutual interactions between ions and the influence of electrical charges, resulting in deviations from ideal behavior. The equation incorporates parameters such as the Debye length, which represents the range of electrostatic interactions, and the Debye-Huckel limiting law, which predicts that as ionic strength increases, the activities of ions decrease. Overall, the Debye-Huckel-Onsager's equation plays a crucial role in understanding the behavior of electrolyte solutions and their properties in various scientific fields, including chemistry, biology, and environmental sciences.
This mind map was published on 14 September 2023 and has been viewed 329 times.