How are CRFs categorised in clinical research?
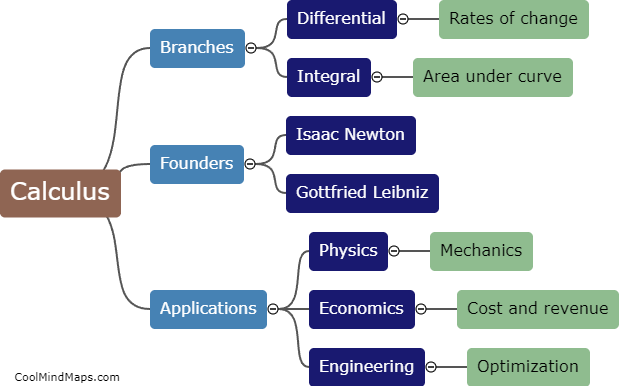
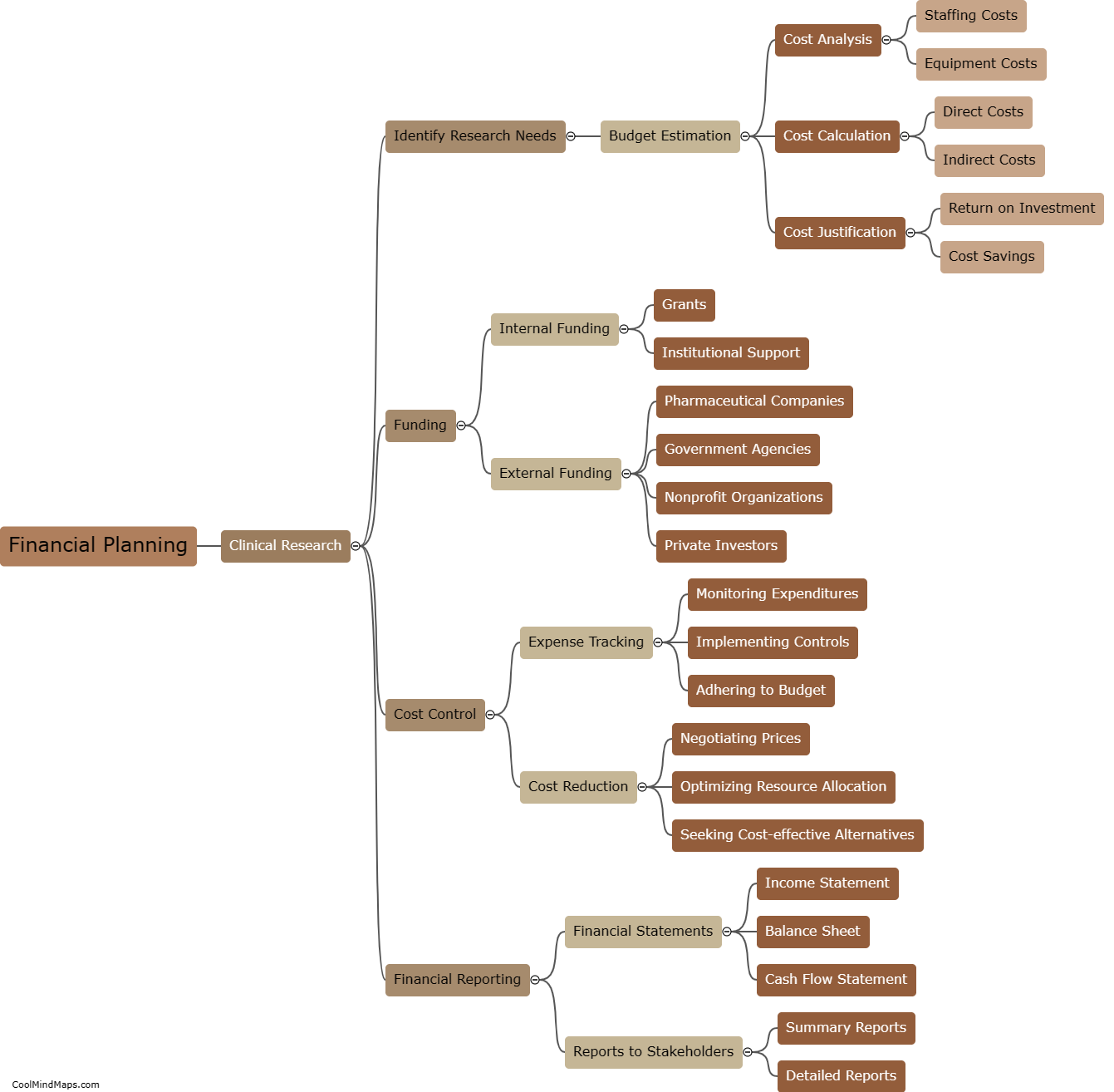
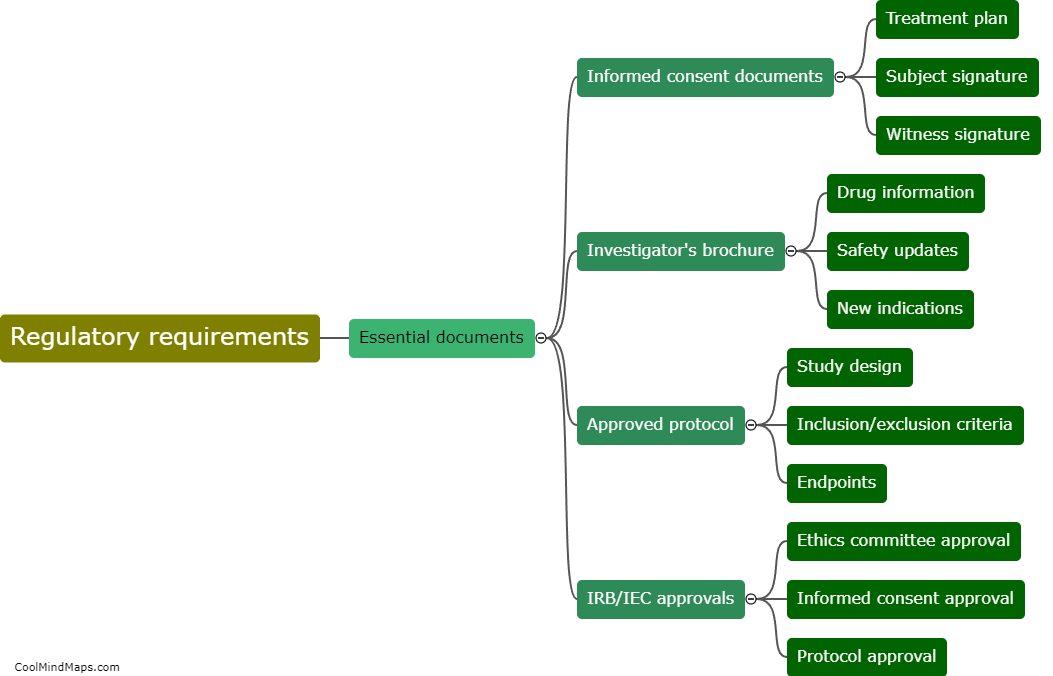
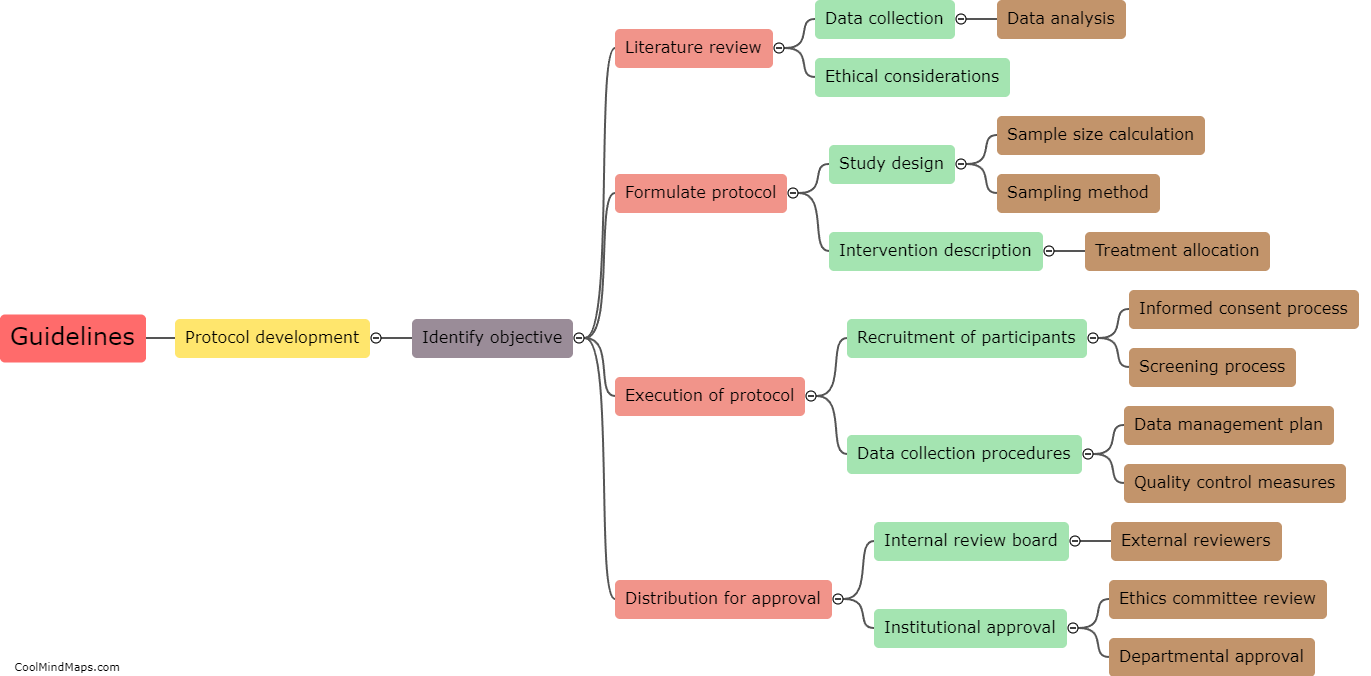
In clinical research, CRFs (Case Report Forms) are categorized based on various factors to ensure efficient data collection and analysis. One of the main classifications is based on the purpose or type of data being collected, such as efficacy CRFs, safety CRFs, or quality of life CRFs. Efficacy CRFs focus on assessing the effectiveness of a treatment or intervention, while safety CRFs document adverse events or side effects. Quality of life CRFs evaluate the impact of a medical intervention on a patient's overall well-being. Another way CRFs are categorized is by their format, including paper-based CRFs and electronic CRFs (eCRFs), with eCRFs becoming more widely used for their ease of data management and remote access capabilities. Overall, proper categorization of CRFs allows researchers to streamline data collection processes, facilitate analysis, and ensure adherence to study objectives.

This mind map was published on 5 December 2023 and has been viewed 86 times.