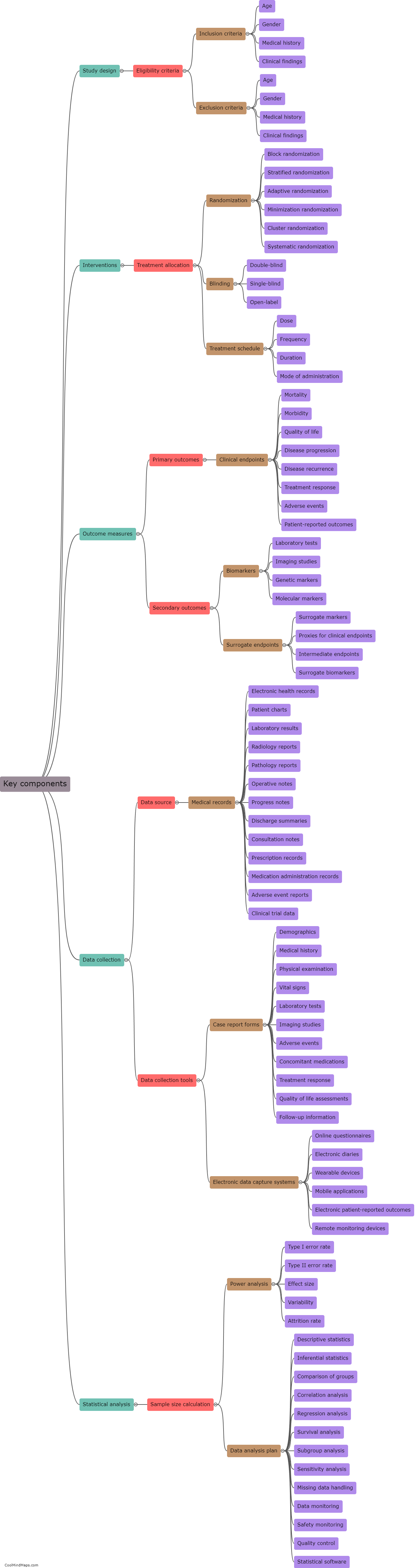
What are the key components of a clinical research protocol?
A clinical research protocol outlines the essential details and procedures of a clinical study. It serves as a blueprint that guides researchers, sponsors, and other stakeholders involved in the study. The key components of a clinical research protocol include background and rationale, objectives, study design and methodology, inclusion and exclusion criteria, sample size determination, assessments and outcome measures, interventions, randomization and blinding procedures, data collection and analysis plan, ethical considerations and informed consent process, monitoring and quality assurance procedures, potential risks and benefits, statistical analysis plan, and finally, the timeline and budget. These components collectively ensure that the study is conducted ethically, follows a well-defined methodology, generates valid and reliable results, and safeguards the rights and well-being of study participants.
This mind map was published on 7 January 2024 and has been viewed 93 times.