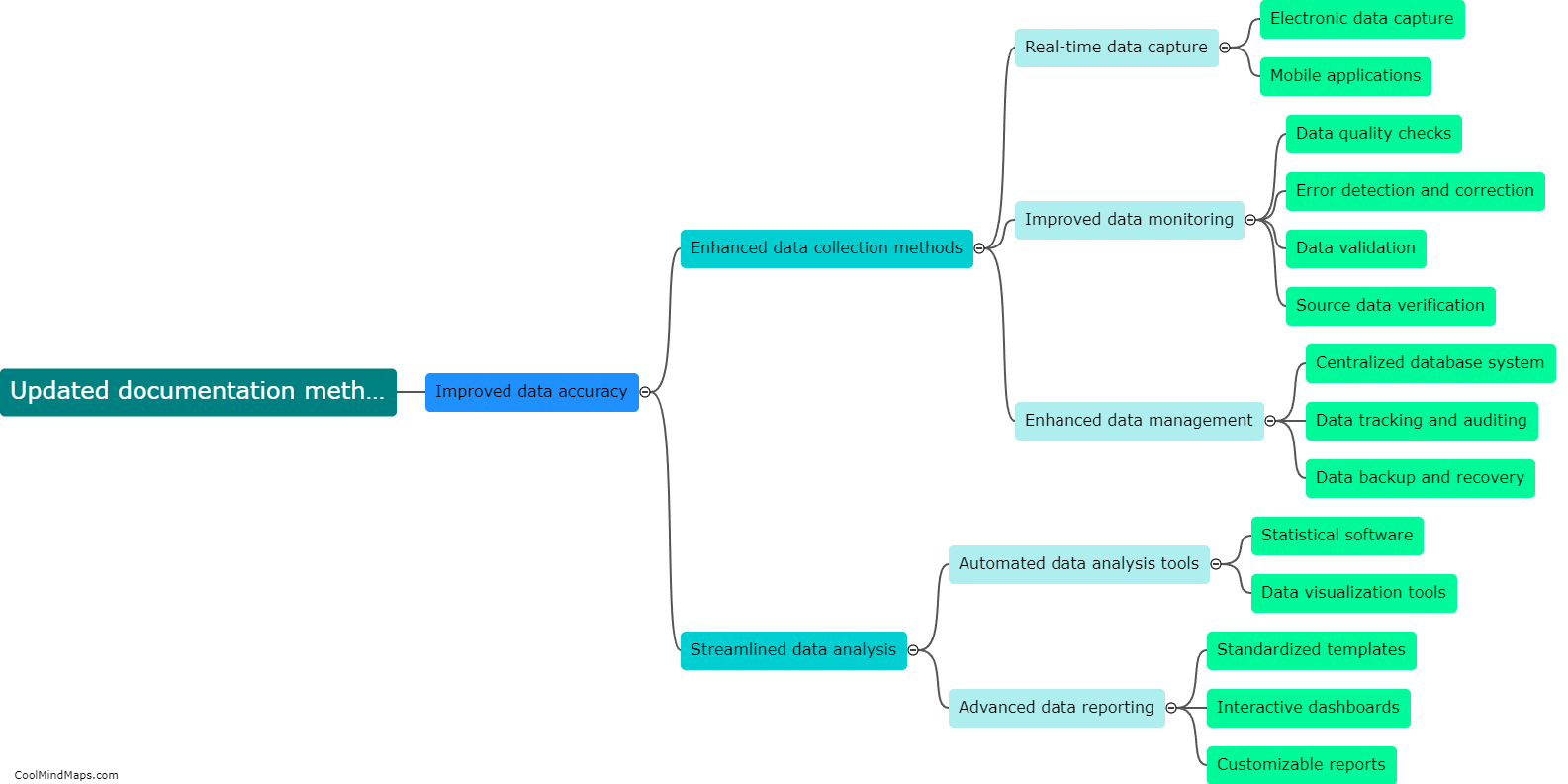
How do updated TMF methods impact the efficiency of clinical trials?
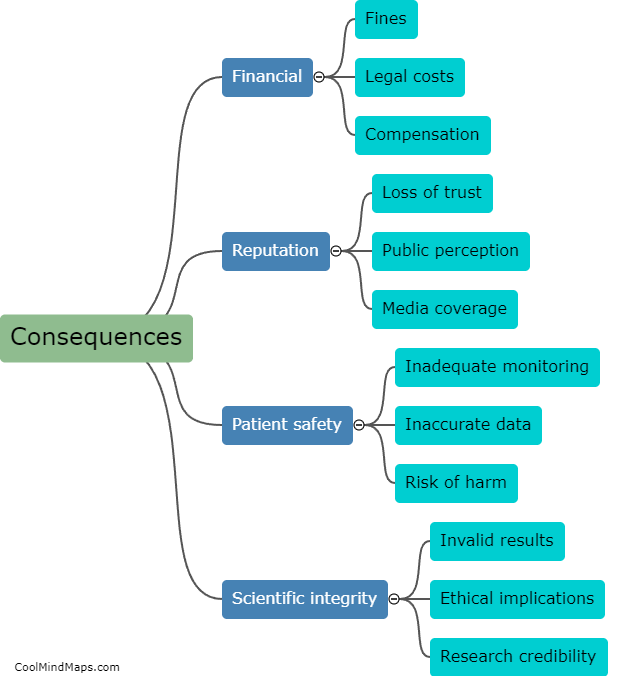
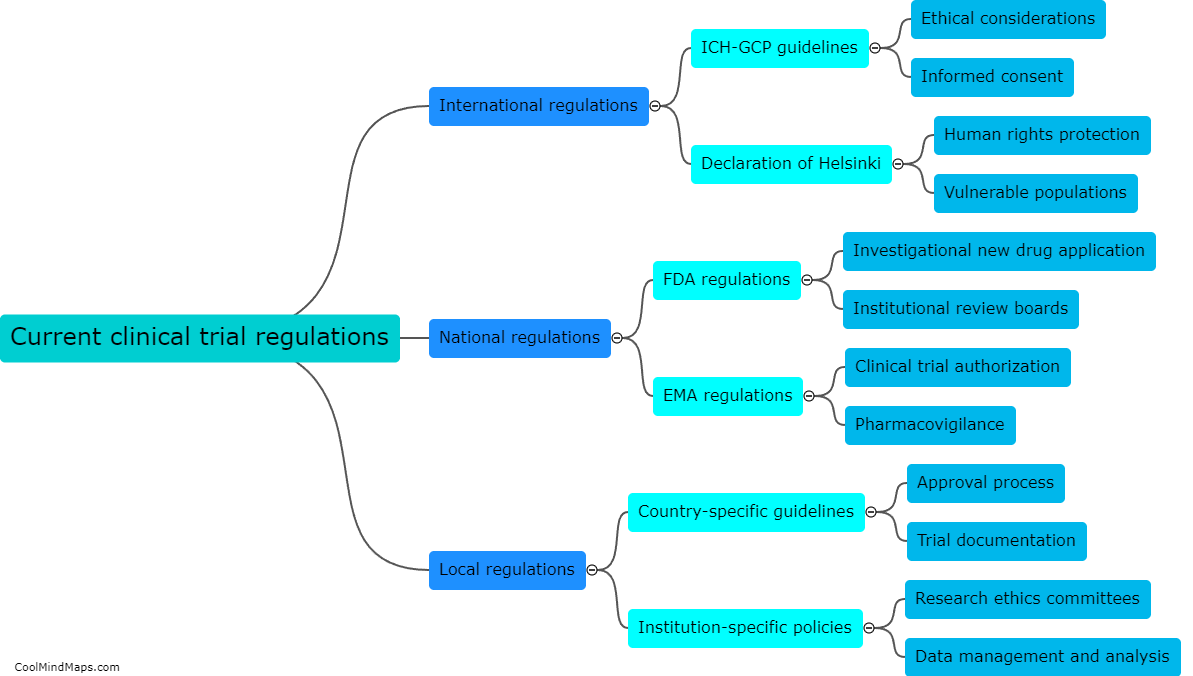
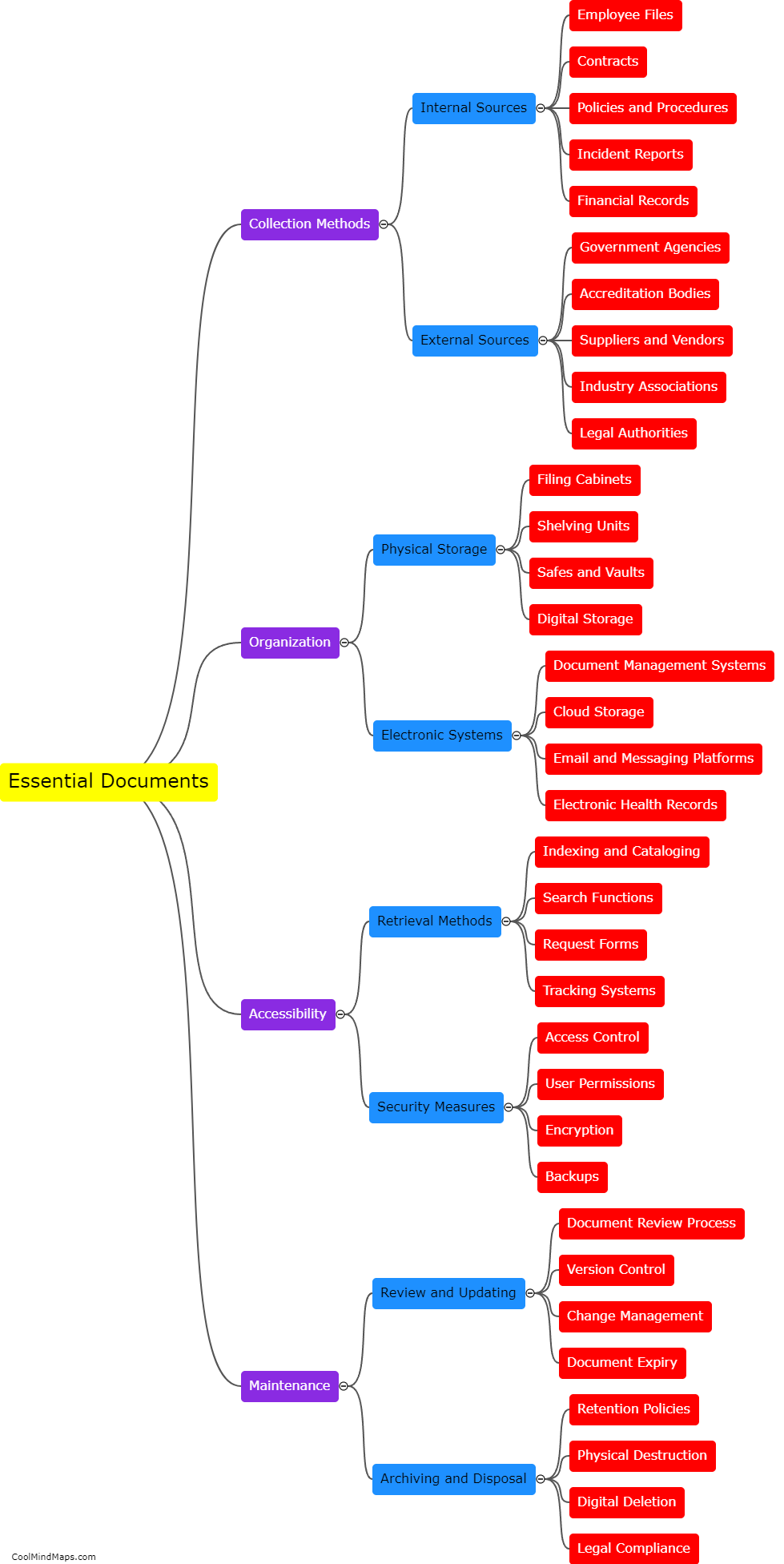
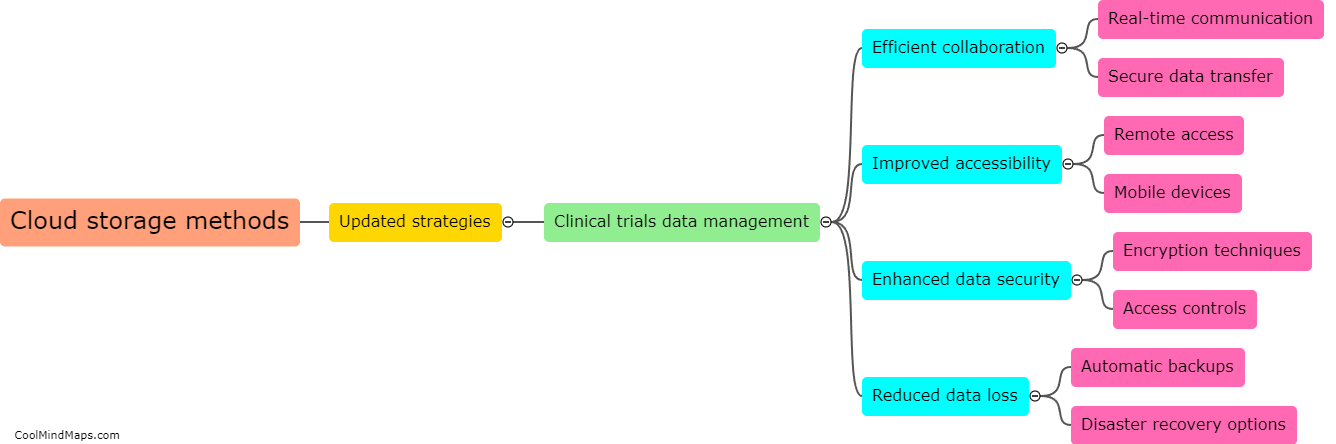
Updated Trial Master File (TMF) methods have a profound impact on the efficiency of clinical trials. TMF is a critical document management system for clinical trial documentation, ensuring compliance with regulatory guidelines. By adopting updated TMF methods, various aspects of a clinical trial can be enhanced. Firstly, streamlined and standardized TMF processes reduce the time and effort spent on administrative tasks, allowing researchers to focus more on study execution. Secondly, updated methods utilize electronic document management systems, enabling real-time access to trial documents from multiple locations, thereby facilitating collaboration among stakeholders. This promotes seamless communication, accelerates decision-making, and ultimately expedites the trial process. Additionally, improved TMF methods ensure higher data integrity, accuracy, and security, aiding in maintaining regulatory compliance and reducing risk. Ultimately, the integration of updated TMF methods significantly boosts the efficiency of clinical trials, leading to faster study completion and potential accelerated patient access to innovative treatments.
This mind map was published on 7 January 2024 and has been viewed 84 times.