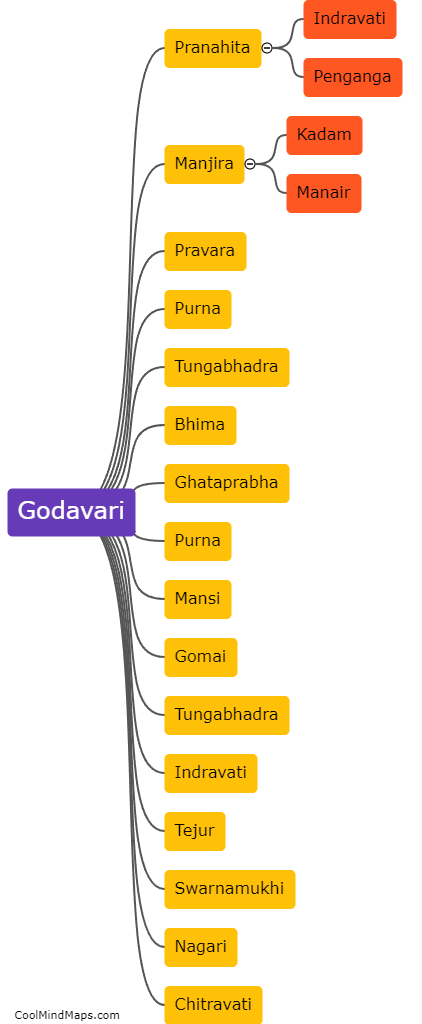
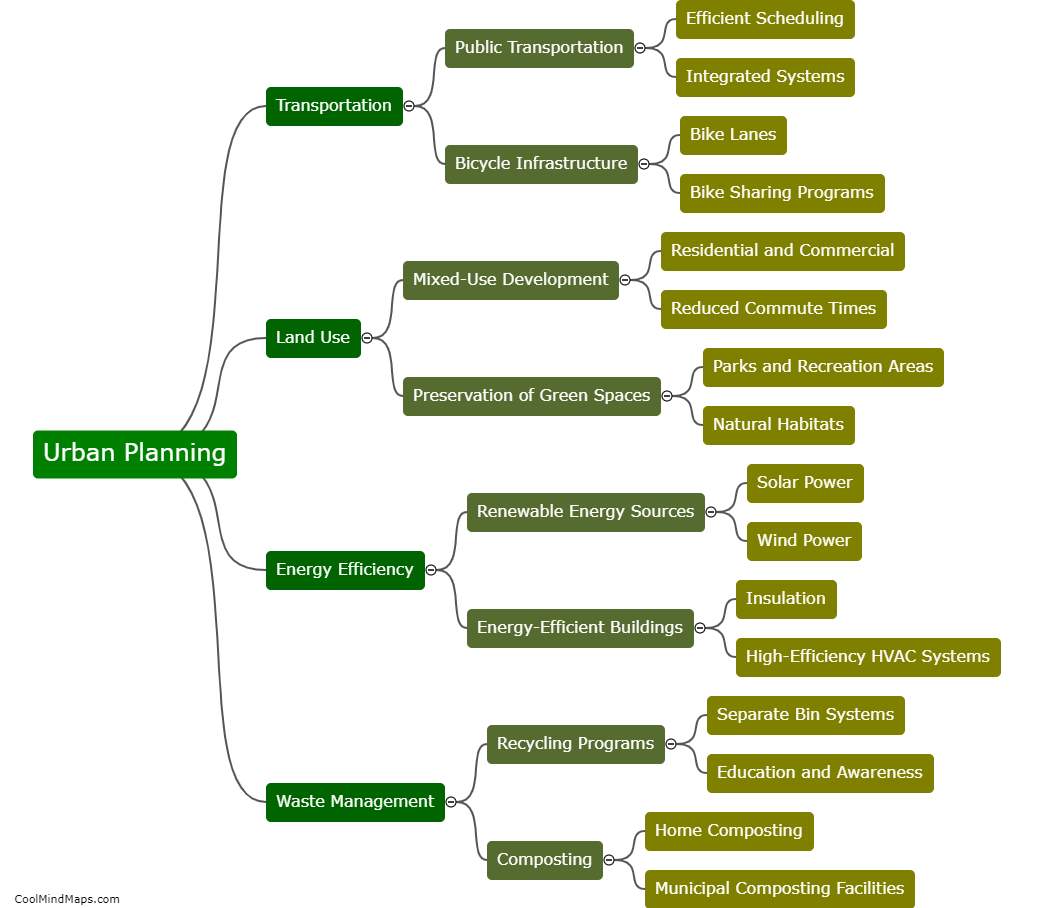
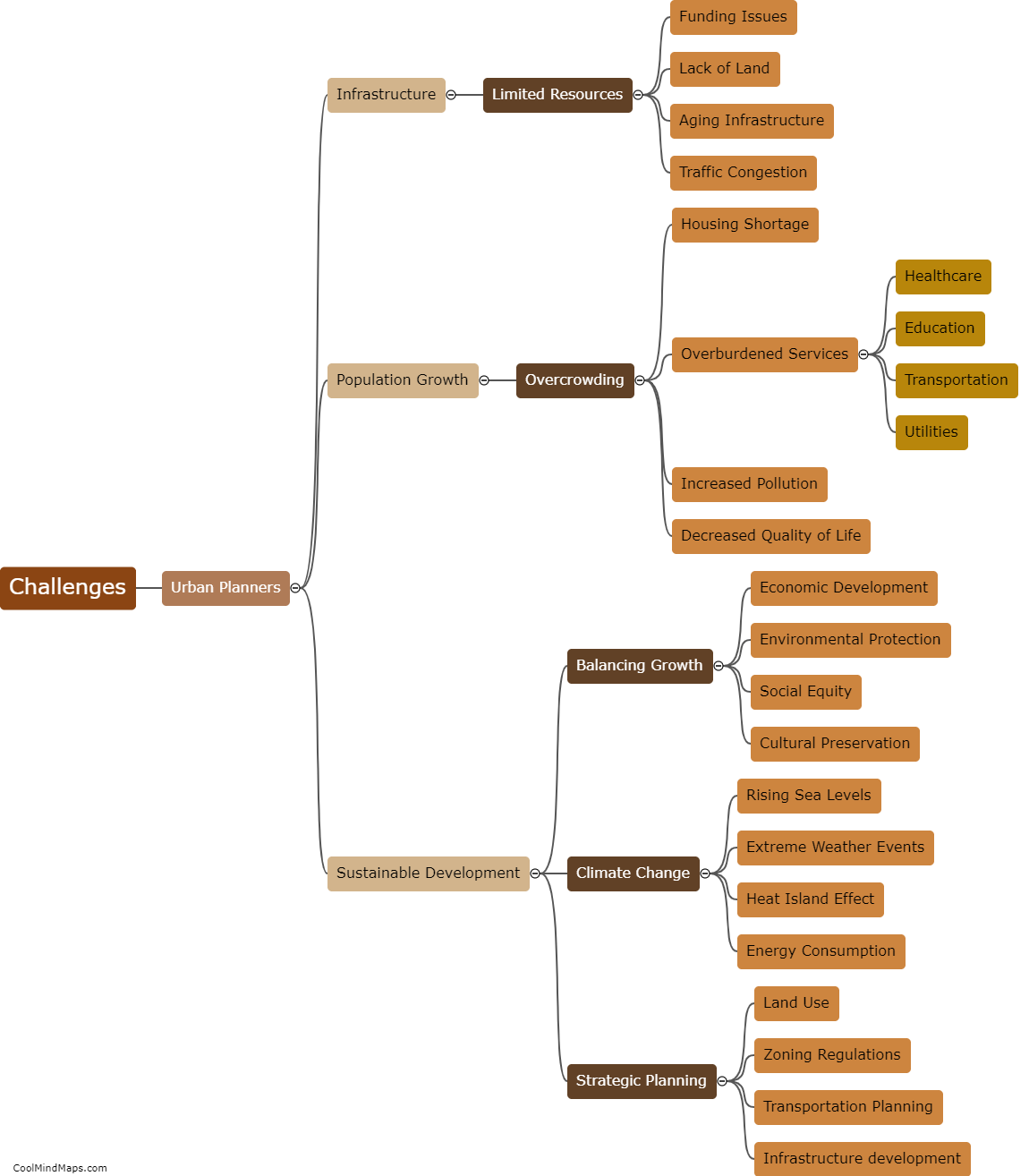
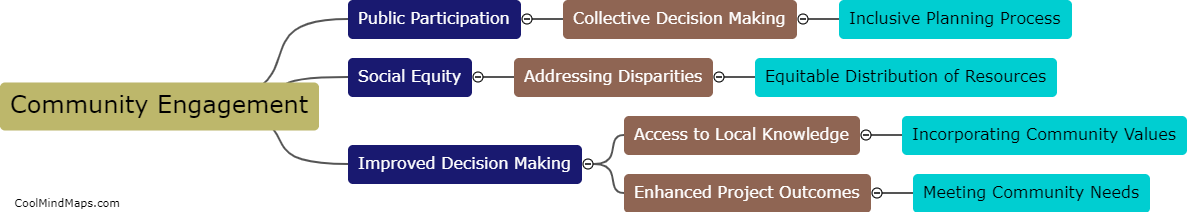
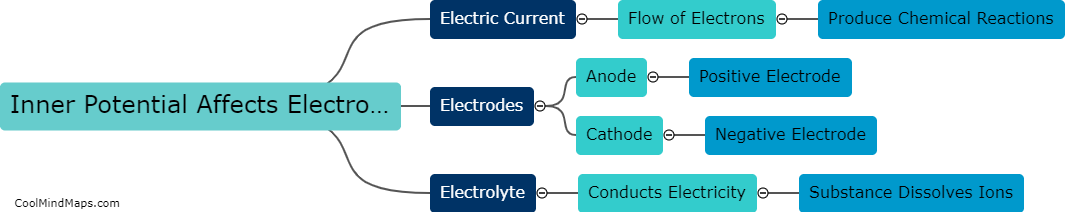
How does inner potential affect electrolysis?
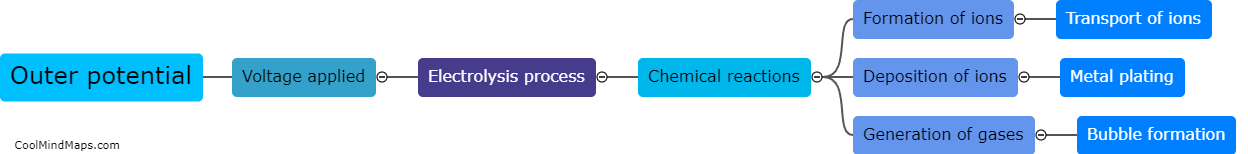
Inner potential can significantly affect the process of electrolysis. Inner potential refers to the electric potential difference between the metal electrode and the solution in which the electrolysis is taking place. When the inner potential is high, it indicates that the metal electrode has a strong tendency to gain or lose electrons, thus affecting the rate of the electrolysis reaction. High inner potential can lead to faster electrolysis as it facilitates the movement of ions and enhances the formation of desired products. On the other hand, a low inner potential may inhibit the electrolysis process, requiring more energy to initiate the reaction. Therefore, understanding and controlling the inner potential is crucial in optimizing the efficiency and effectiveness of electrolysis processes, particularly in industrial applications such as metal plating or water splitting for hydrogen production.
This mind map was published on 25 November 2023 and has been viewed 113 times.