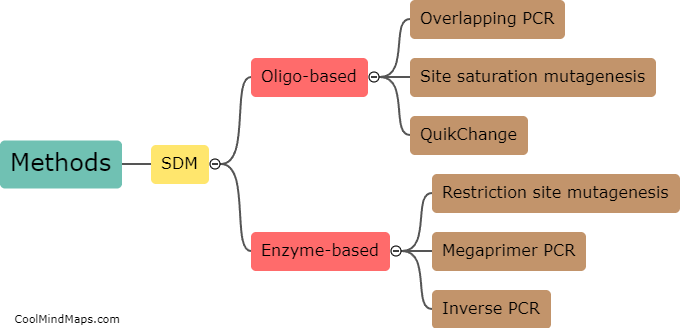
What are the different types of electrochemical electrodes?
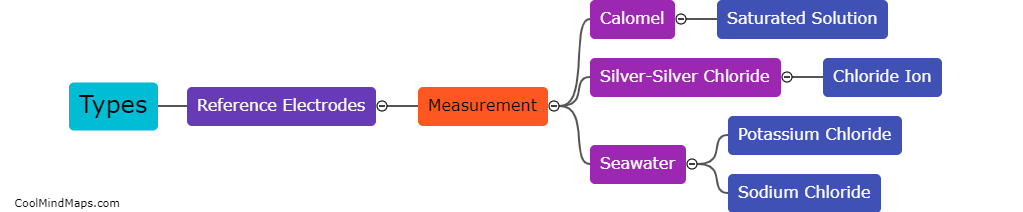
There are various types of electrochemical electrodes used in chemical and biological analysis. The most commonly used electrode is the reference electrode, which provides a stable potential against which the working electrode is measured. Another important electrode is the working electrode, which is the site where the electrochemical reaction occurs. This electrode can be made from various materials like glassy carbon, platinum, or gold, depending on the desired application. Additionally, there are auxiliary or counter electrodes, which complete the electrical circuit and balance the current flow during the electrochemical reaction. These electrodes are typically made from inert materials like platinum or graphite. Overall, the choice of electrode material and type depends on the specific electrochemical analysis or application at hand.

This mind map was published on 12 December 2023 and has been viewed 88 times.