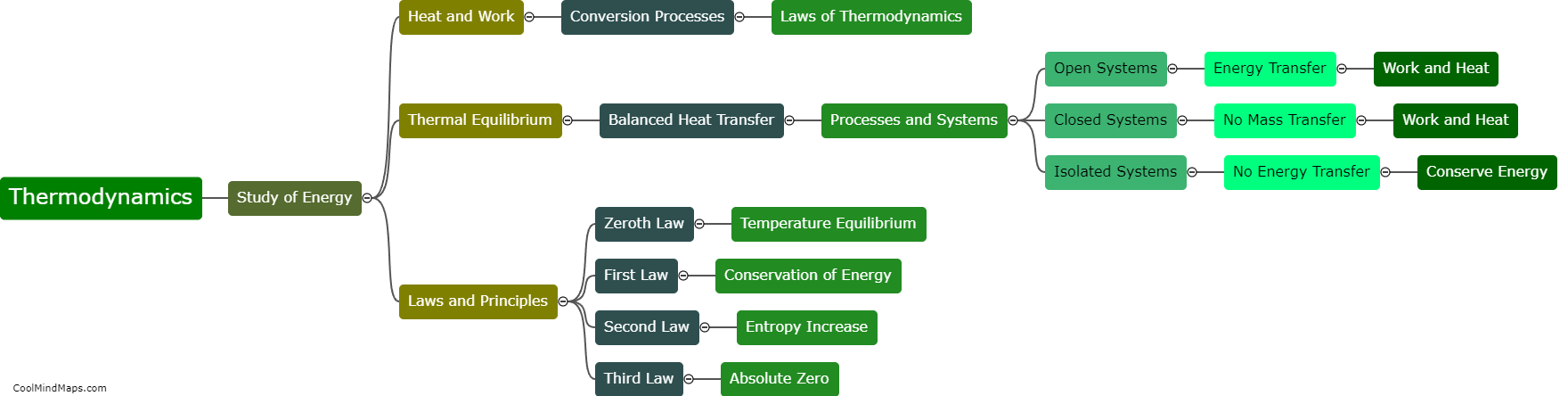
What is the zeroth law of thermodynamics?
The zeroth law of thermodynamics states that if two systems are in thermal equilibrium with a third system, then they are also in thermal equilibrium with each other. This law establishes the concept of temperature and allows for the measurement and comparison of temperatures between different objects or systems. It serves as a foundational principle in thermodynamics, enabling the understanding and analysis of heat transfer and energy flow in various physical and engineering systems. The zeroth law helps establish a reference point for temperature, allowing for the development of temperature scales and the formulation of fundamental principles in thermodynamics.

This mind map was published on 4 September 2023 and has been viewed 100 times.