What is a rate of reaction in chemistry?
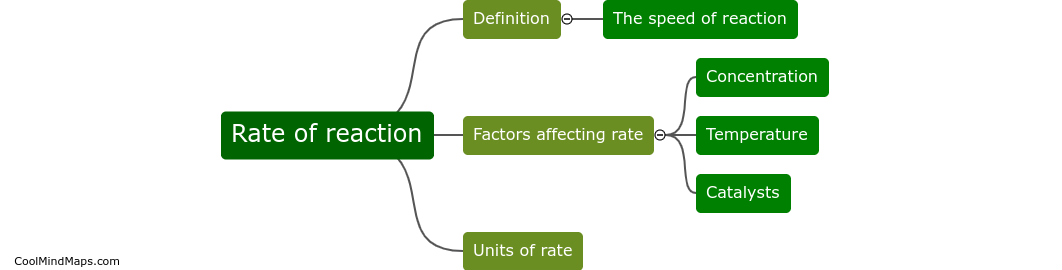
In chemistry, the rate of reaction refers to how quickly a chemical reaction occurs. It is a measure of the change in concentration of reactants or products over a certain period of time. Factors that can affect the rate of reaction include temperature, concentration of reactants, pressure, surface area, and the presence of catalysts. Understanding and manipulating reaction rates is crucial in various industries and research fields to optimize processes and produce desired products efficiently.
This mind map was published on 20 March 2024 and has been viewed 161 times.