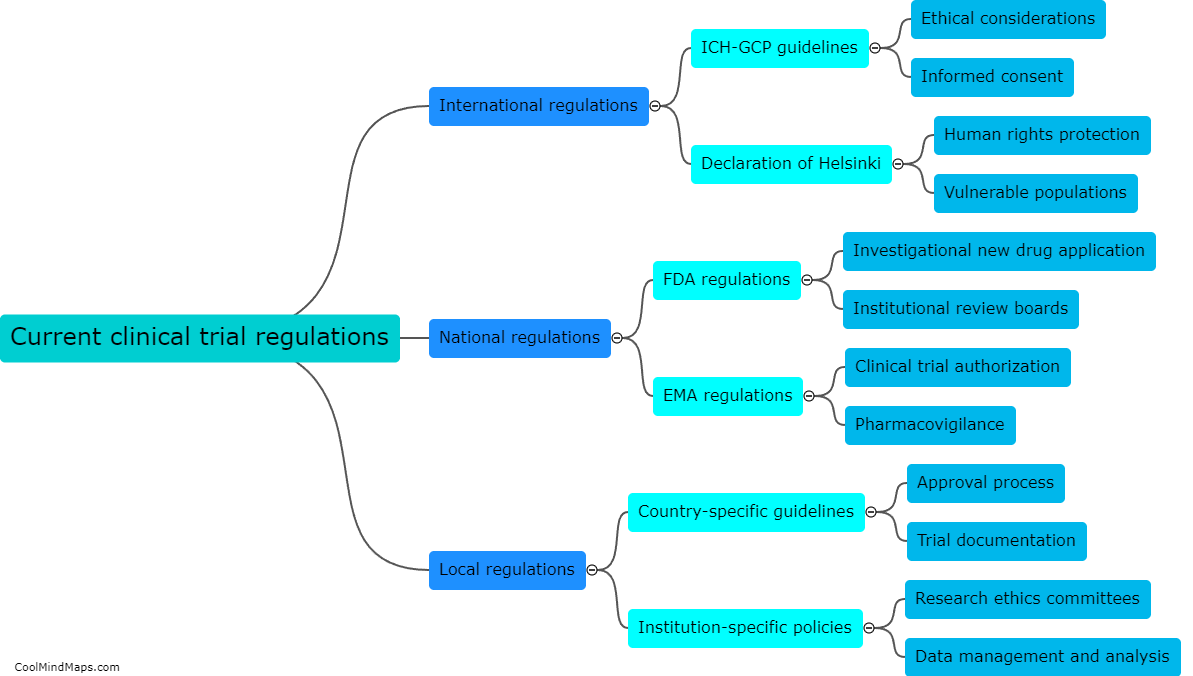
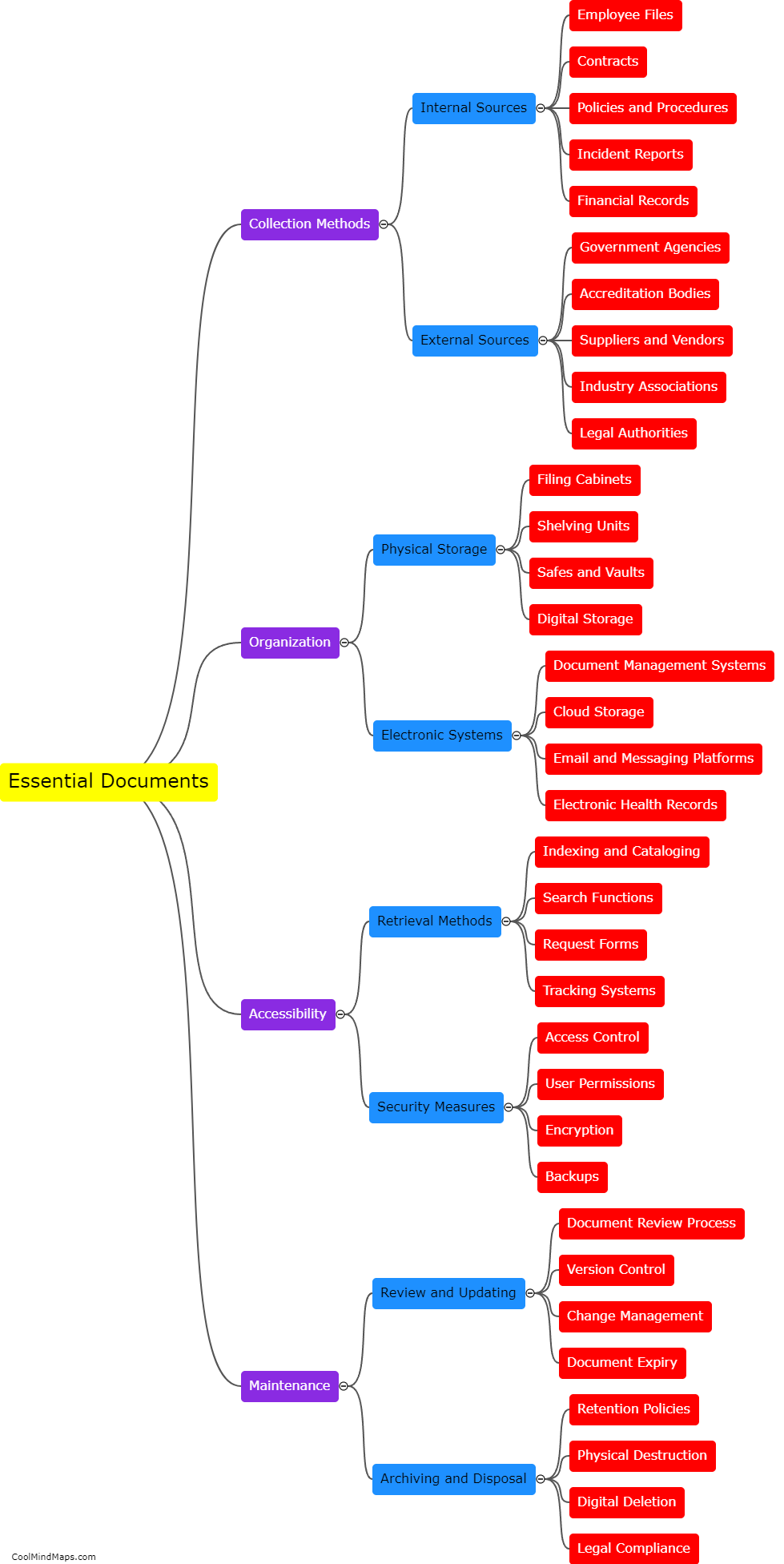
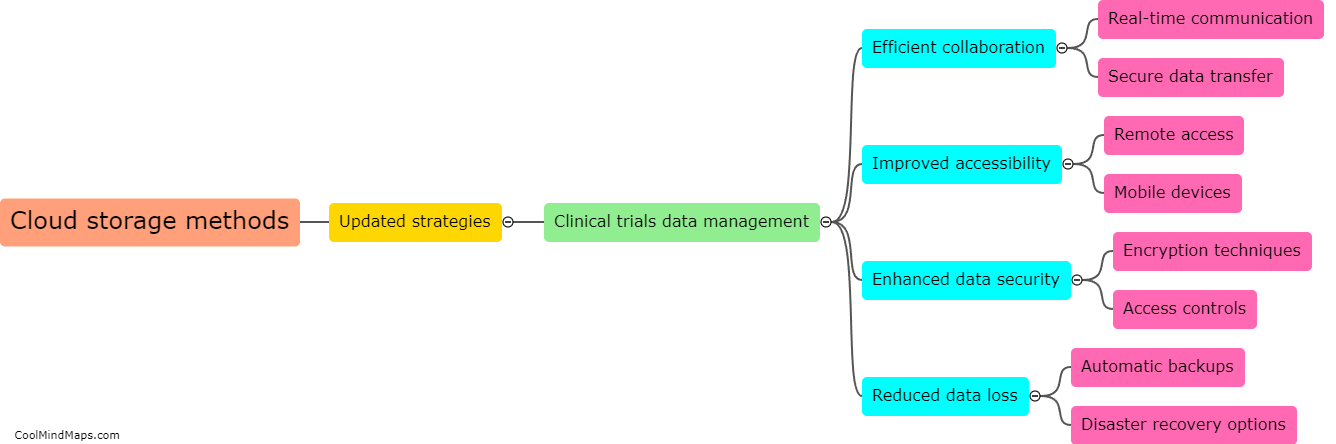
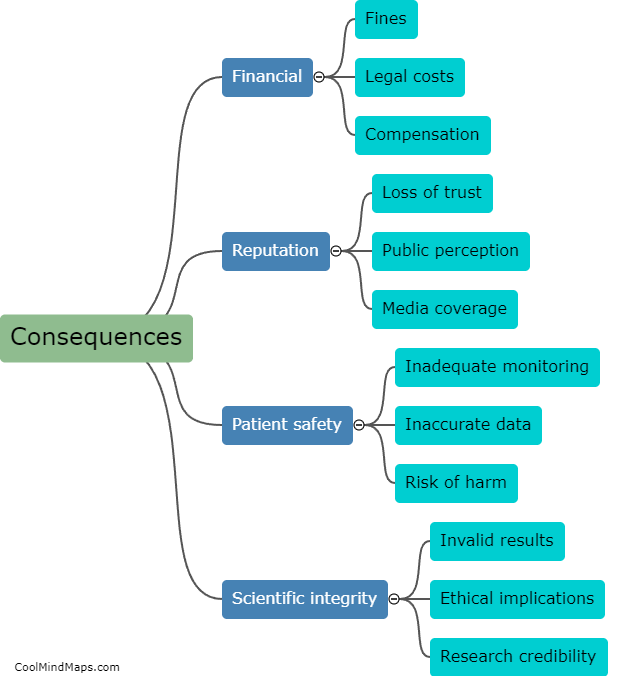
What are the consequences of non-compliance with clinical trial regulations?
Non-compliance with clinical trial regulations can have significant consequences for both the researchers conducting the trials and the participants involved. From a legal perspective, non-compliance can result in penalties and fines for the researchers, potentially leading to reputational damage for their institution or organization. Additionally, non-compliance can compromise the validity and reliability of the trial's data, potentially rendering the results unusable or unreliable. This can not only harm the participants who volunteered for the trial but also hinder scientific progress and the development of new treatments or interventions. Non-compliance can also erode public trust in the clinical research field and diminish the credibility of future trials. Consequently, it is crucial that researchers comply with all relevant regulations and ethical guidelines to ensure the integrity and safety of clinical trials.
This mind map was published on 7 January 2024 and has been viewed 89 times.