What is the difference between an acid and a base?
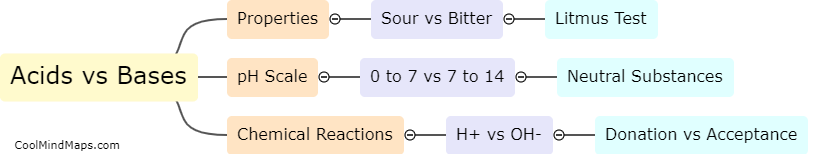
Acids and bases are two types of chemicals that exhibit opposite properties. Acids are substances that donate hydrogen ions (H+) when dissolved in water, resulting in a lower pH value, typically less than 7, indicating an acidic solution. Bases, on the other hand, donate hydroxide ions (OH-) when dissolved in water, leading to a higher pH value, normally greater than 7, indicating a basic or alkaline solution. Acids have a sour taste and can react with metals to release hydrogen gas, while bases have a bitter taste and feel slippery to touch. These differences reflect their unique chemical properties and determine their behavior in various chemical reactions, particularly in acid-base reactions.
This mind map was published on 18 April 2023 and has been viewed 93 times.