How does uranium decay into other elements?
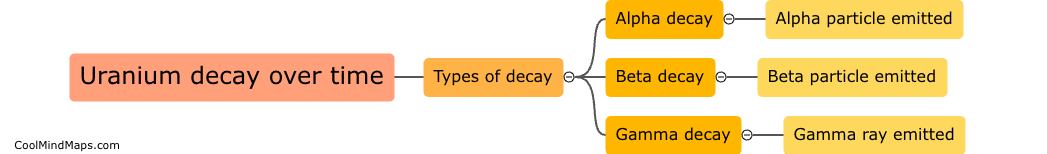
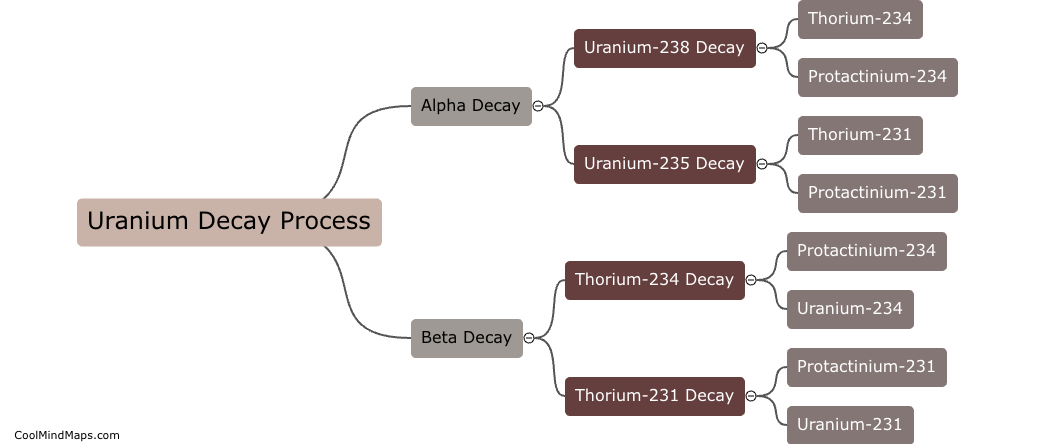
Uranium decays into other elements through a series of radioactive decay processes. The most common form of uranium, uranium-238, undergoes a series of alpha and beta decays to eventually transform into stable lead-206. During this decay process, uranium-238 releases alpha particles, which are essentially helium nuclei, and beta particles, which are high-energy electrons. Through the emission of these particles, uranium-238 loses protons and neutrons, transforming into different elements until it reaches stability as lead-206.
This mind map was published on 22 March 2024 and has been viewed 91 times.