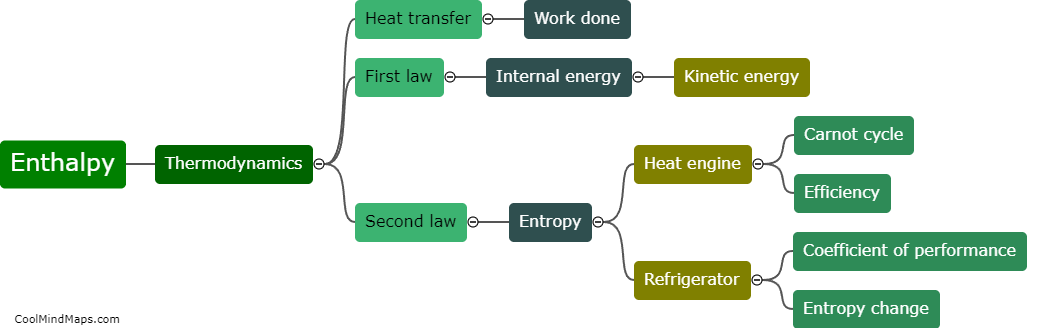
How does enthalpy relate to thermodynamics?
Enthalpy is a fundamental concept in thermodynamics, a branch of science that deals with the study of energy and its transformations. Specifically, enthalpy is a measure of the total heat content of a system at constant pressure. It incorporates both the internal energy and the pressure-volume work of a system, making it a valuable tool for studying energy changes during chemical reactions or phase transitions. Enthalpy plays a crucial role in the formulation of key thermodynamic equations, such as the first law of thermodynamics, which states that energy cannot be created or destroyed, only transferred or transformed. By understanding and manipulating enthalpy, scientists and engineers can predict and control energy transfer and transformation processes, from the design of chemical reactions to the efficient operation of power plants.
This mind map was published on 19 September 2023 and has been viewed 115 times.