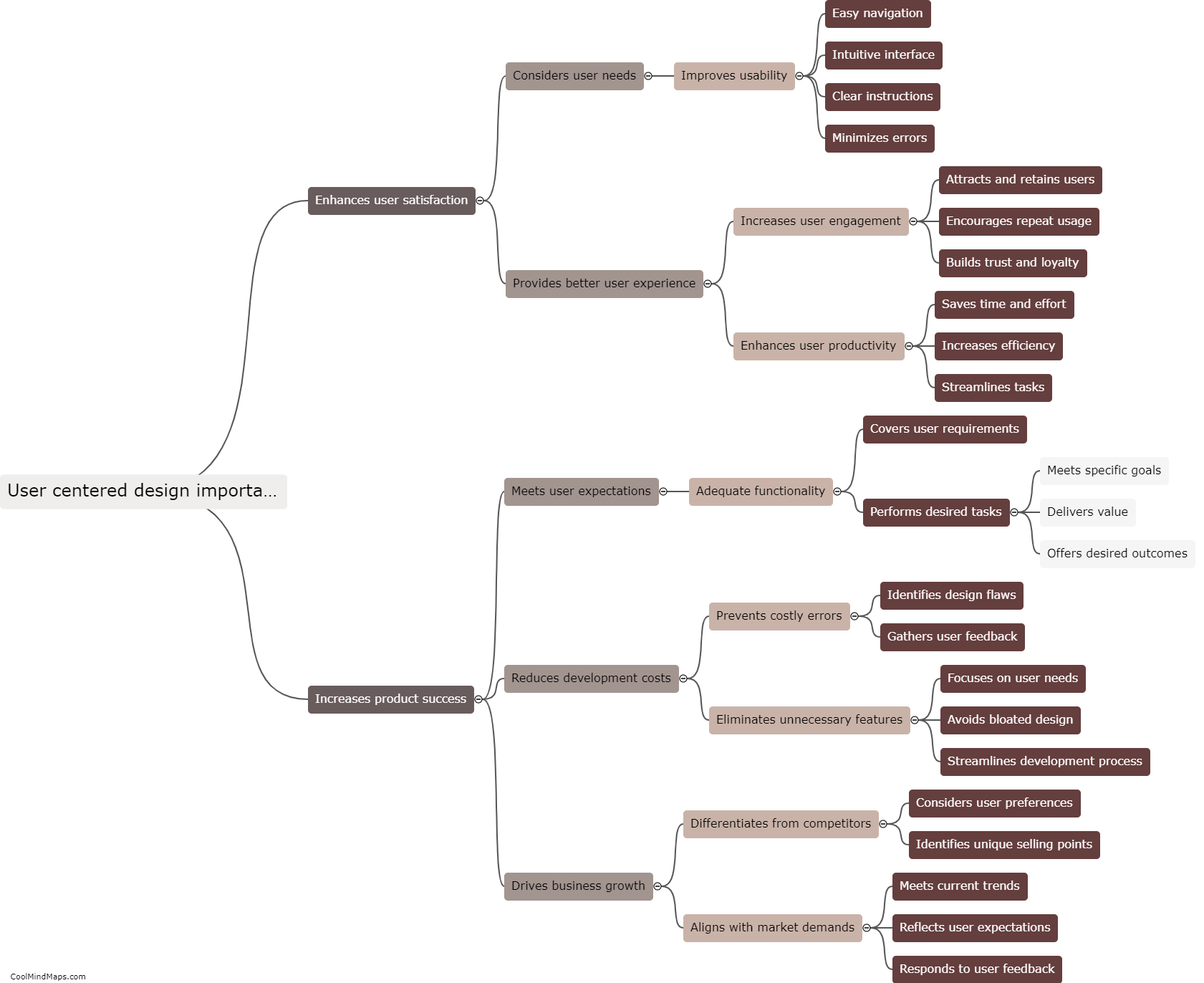
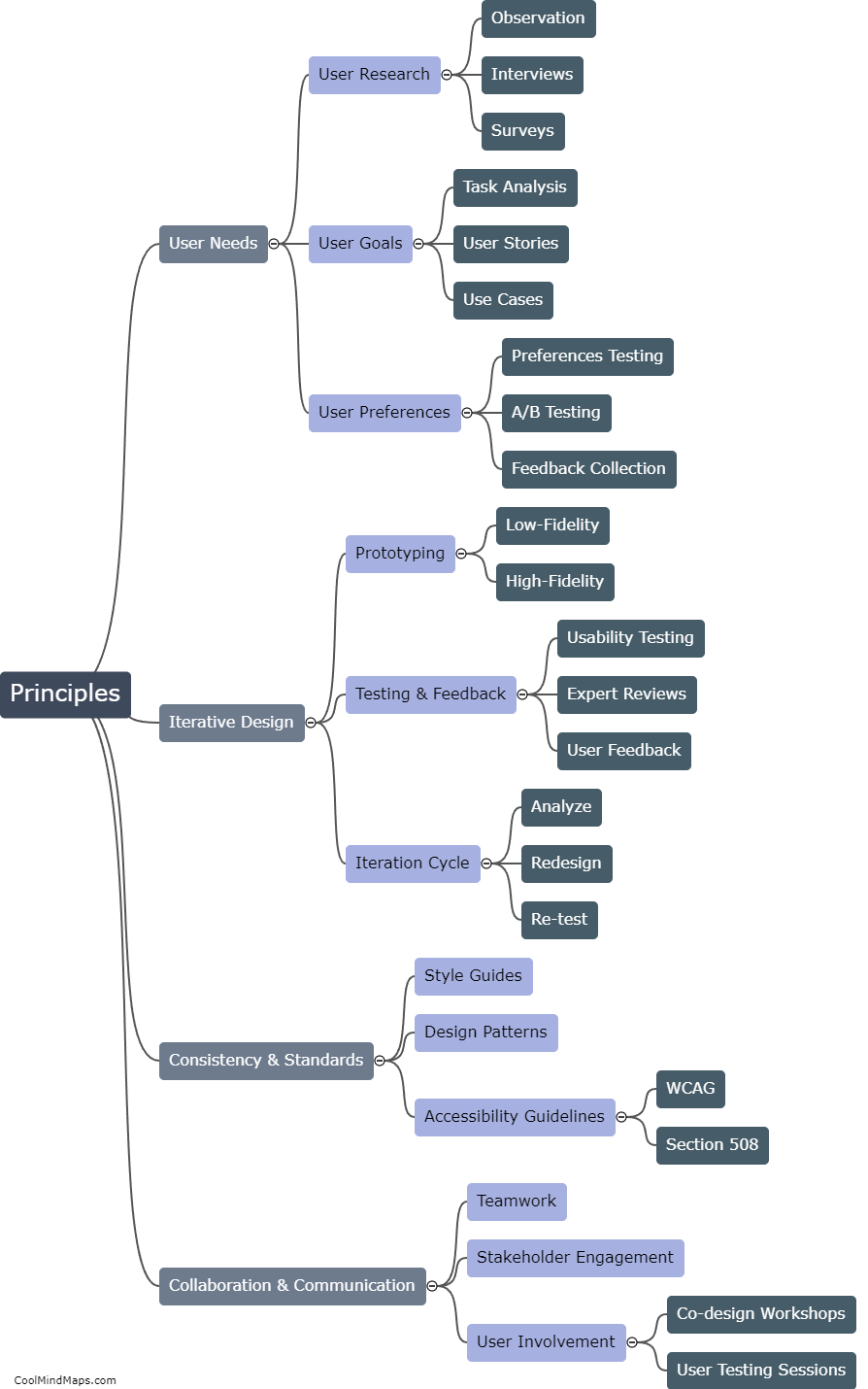
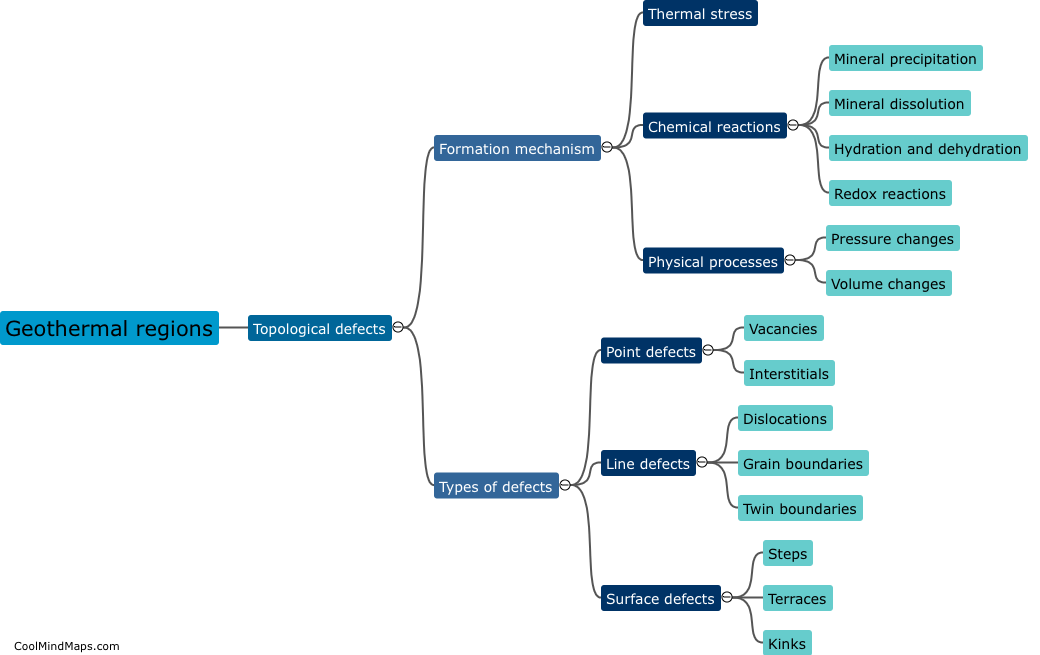
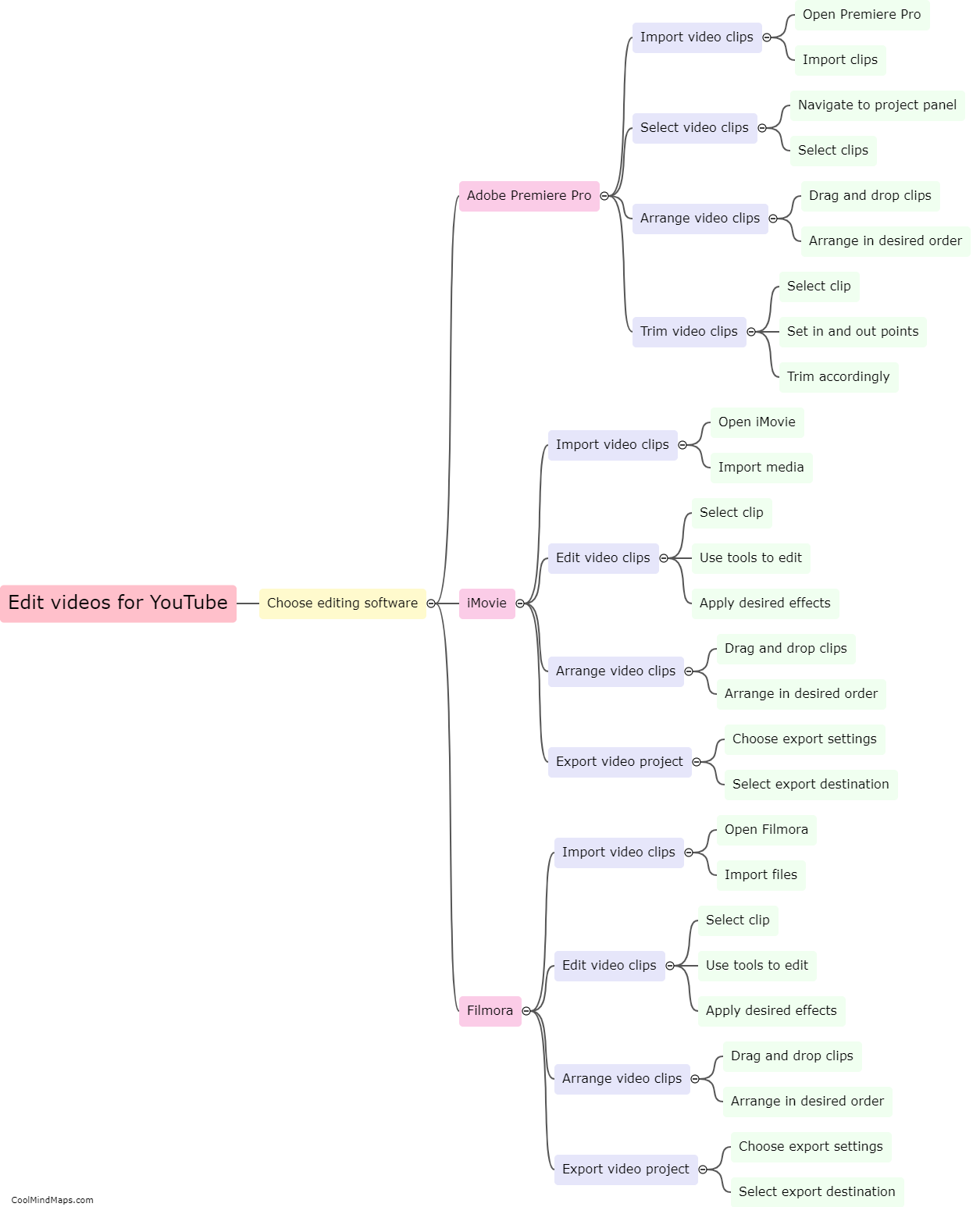
How does the law of conservation of mass apply to systems?
The law of conservation of mass states that matter cannot be created or destroyed in a closed system. This fundamental principle in chemistry and physics applies to all systems, whether they are chemical reactions, physical processes, or even the entire universe. In any system, no matter the complexity or scale, the total mass of all the substances involved remains constant. This means that the amount of mass on the input side must equal the amount of mass on the output side. While matter may undergo changes in form, such as solid to liquid or gas, the total mass of the system remains unchanged. It is through the application of this law that scientists are able to accurately analyze and quantify the behavior and outcome of various processes and reactions.

This mind map was published on 9 October 2023 and has been viewed 90 times.