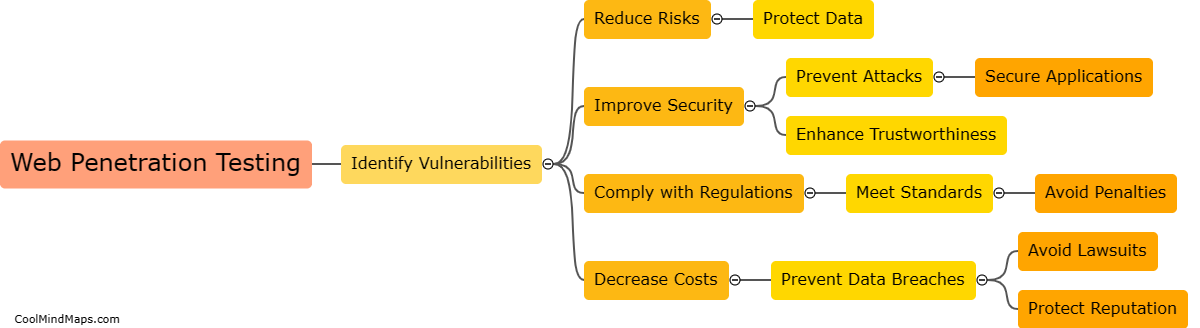
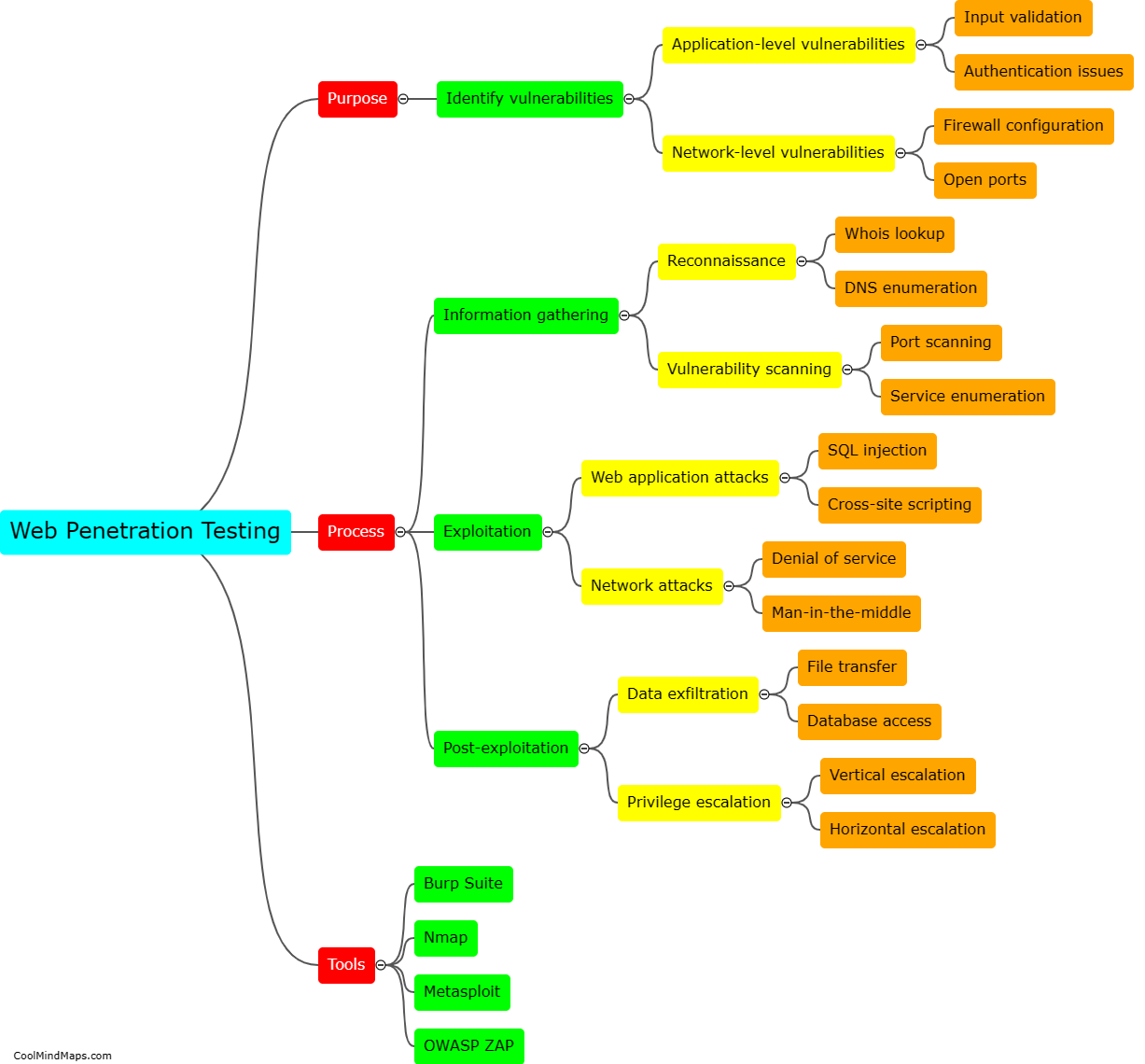
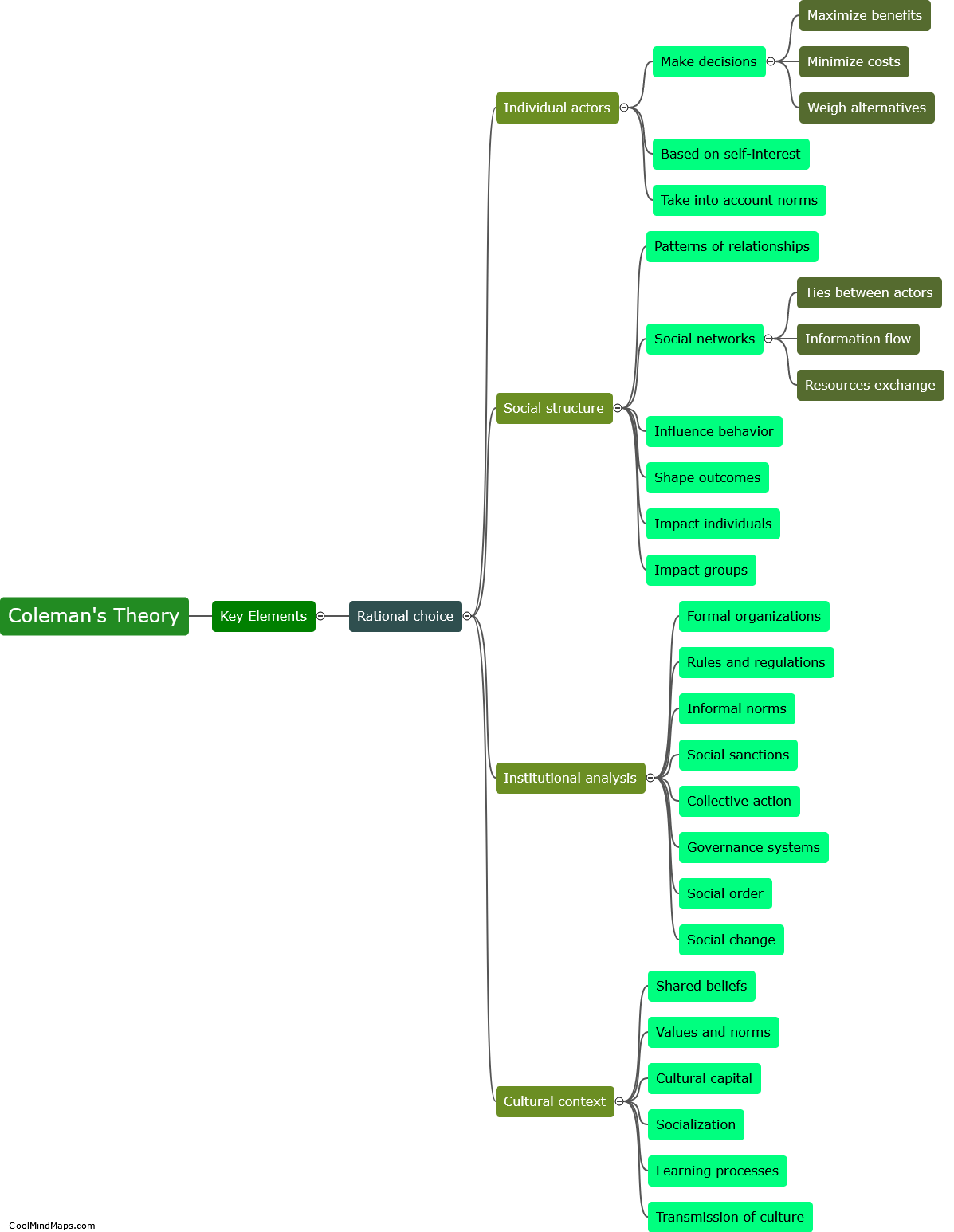
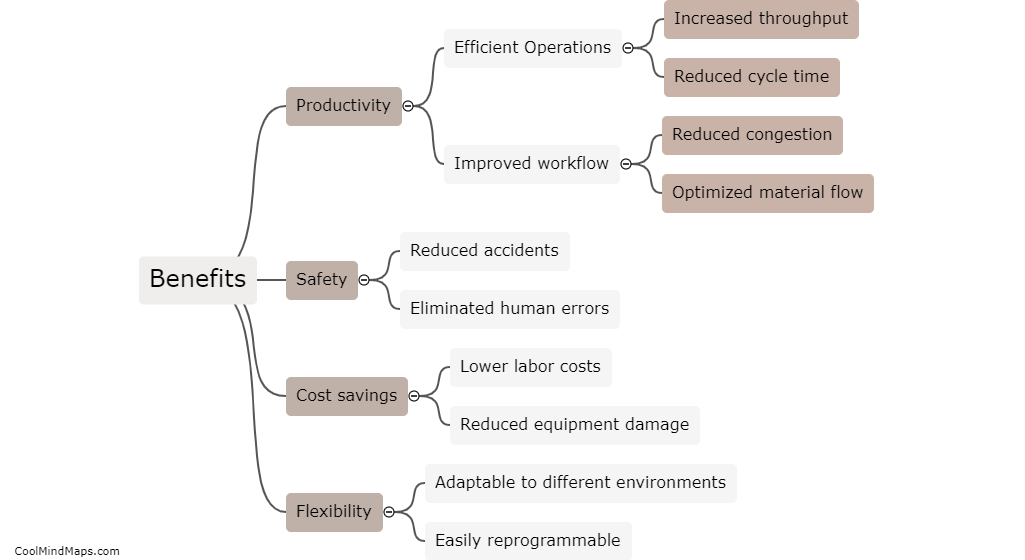
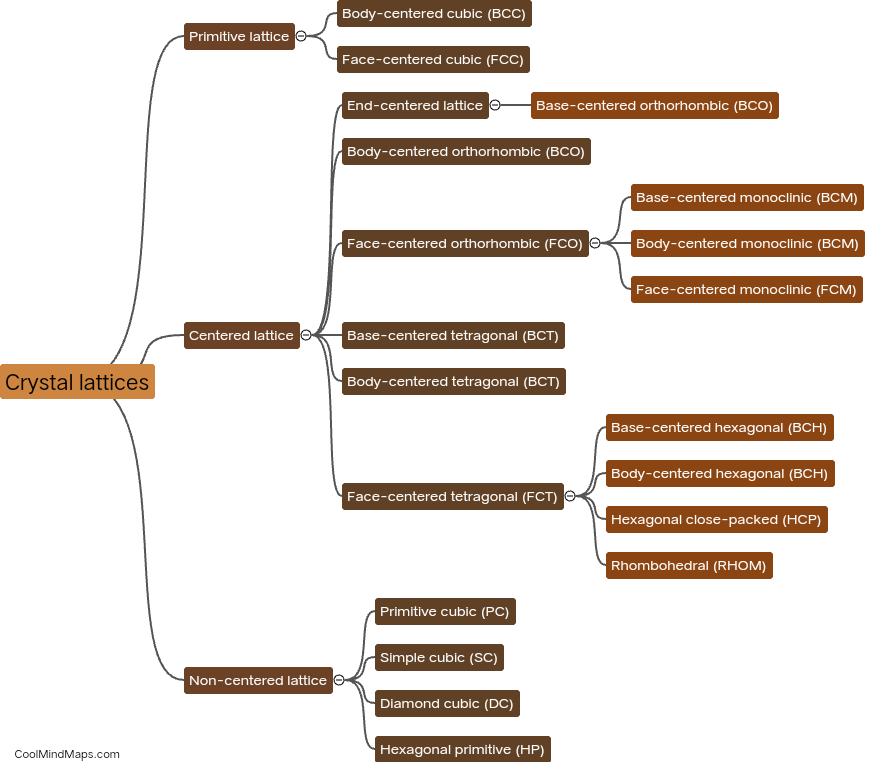
What are the different types of crystal lattices?
Crystal lattices are repeating geometric arrangements of atoms or ions in a crystal structure. There are several different types of crystal lattices that can form, depending on the nature of the crystal's constituents and the conditions under which it is formed. The most common types of crystal lattices include the simple cubic lattice, where atoms are arranged at the corners of a cube; the body-centered cubic lattice, where atoms are arranged at the corners and center of a cube; and the face-centered cubic lattice, where atoms are arranged at the corners and center of each face of a cube. Other types of crystal lattices include the hexagonal close-packed lattice and the orthorhombic lattice. Each lattice structure has unique properties that contribute to a crystal's physical and chemical characteristics.
This mind map was published on 11 January 2024 and has been viewed 93 times.