How are paper CRFs and e-CRFs used in clinical trials?
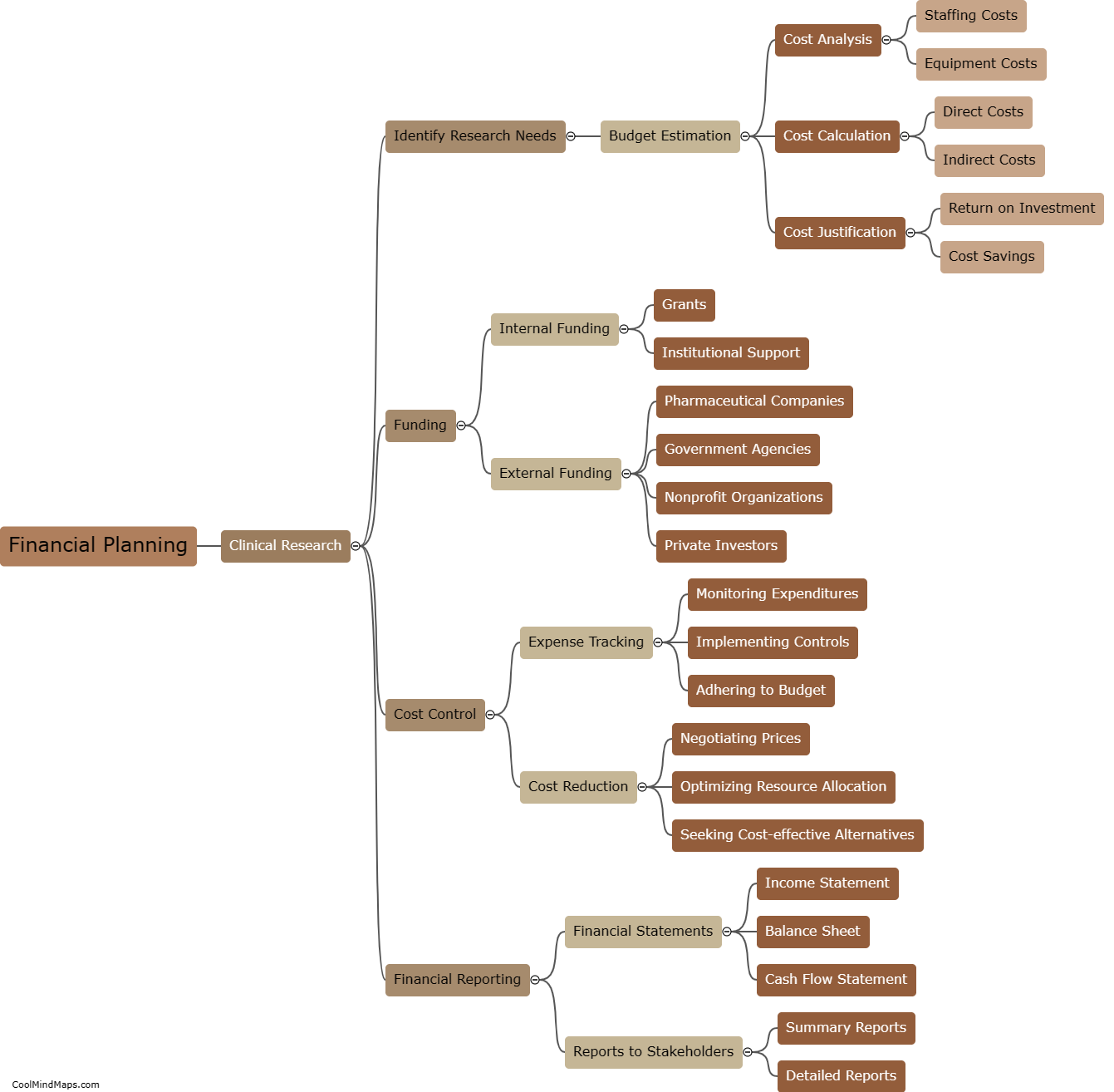
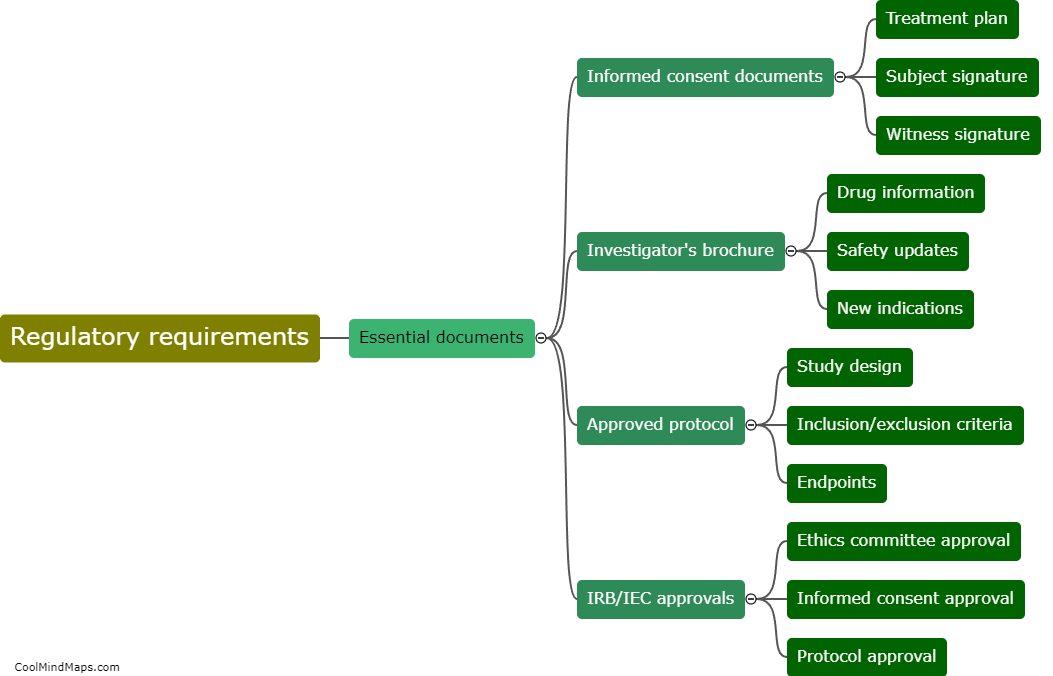
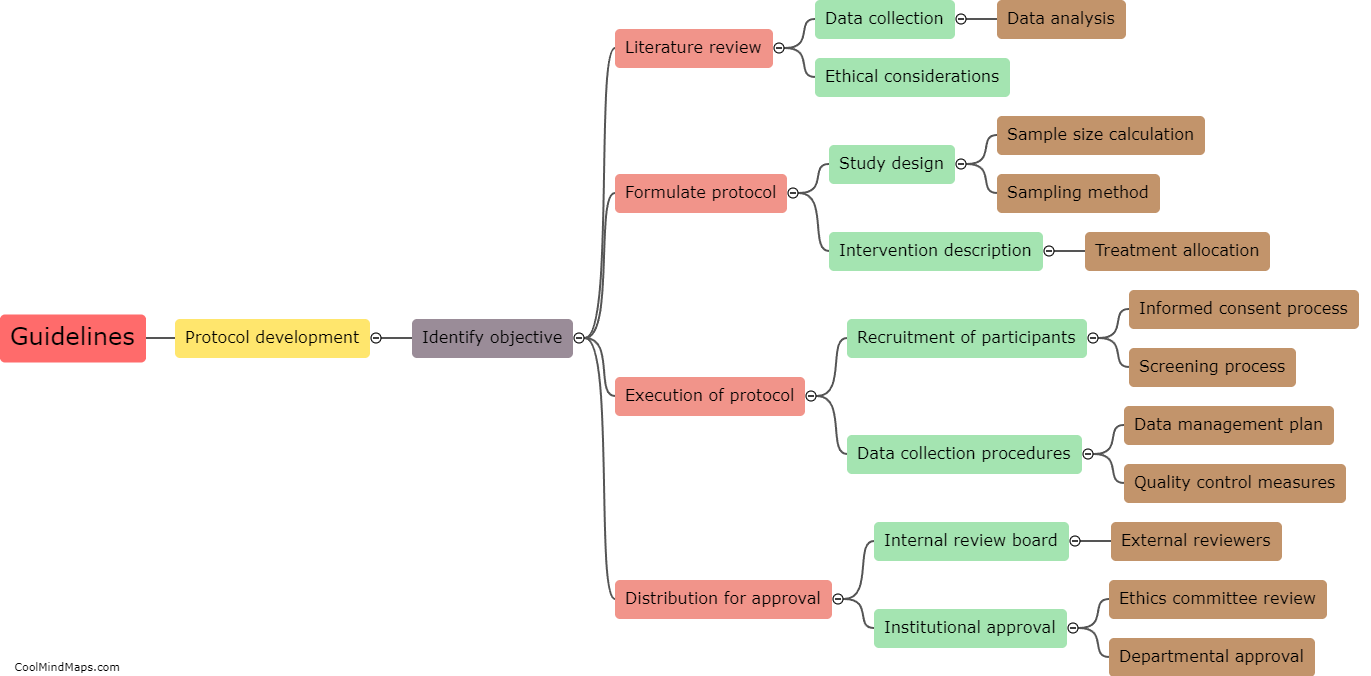
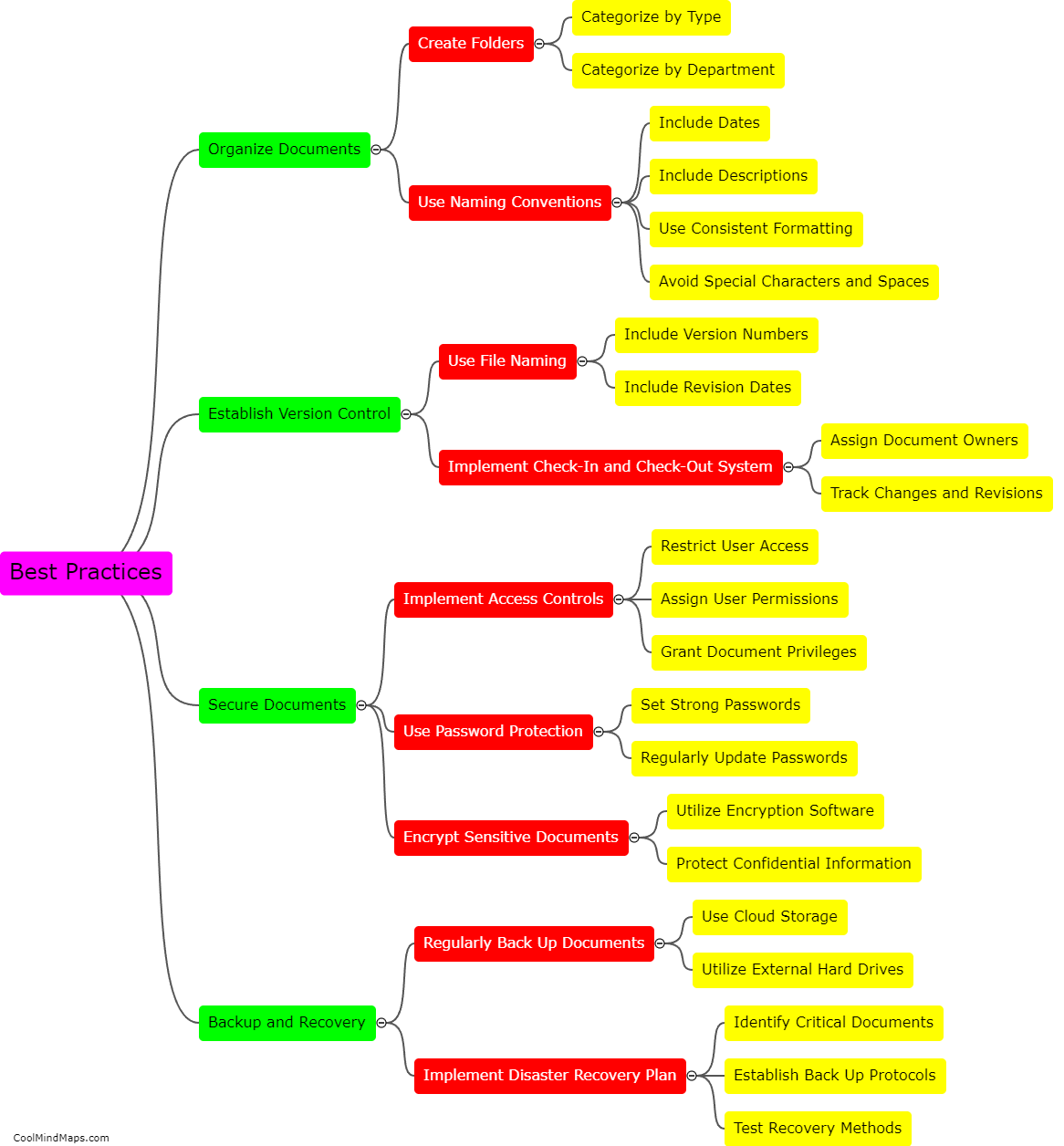
Paper Case Report Forms (CRFs) and electronic Case Report Forms (e-CRFs) are both utilized in clinical trials to gather essential data about patients and treatments. Paper CRFs, as traditional forms, involve printed sheets that clinicians complete manually with the patients' information, study variables, and treatment outcomes. These physical forms are then gathered and entered into electronic databases for analysis. On the other hand, e-CRFs are digital versions that clinicians can fill out directly into a secure online system. These digital forms automatically capture and store data electronically, enabling real-time data collection and data management throughout the trial. The choice between these formats often depends on factors such as trial design, resources, preferences, and the complexity of data collection requirements.

This mind map was published on 5 December 2023 and has been viewed 81 times.