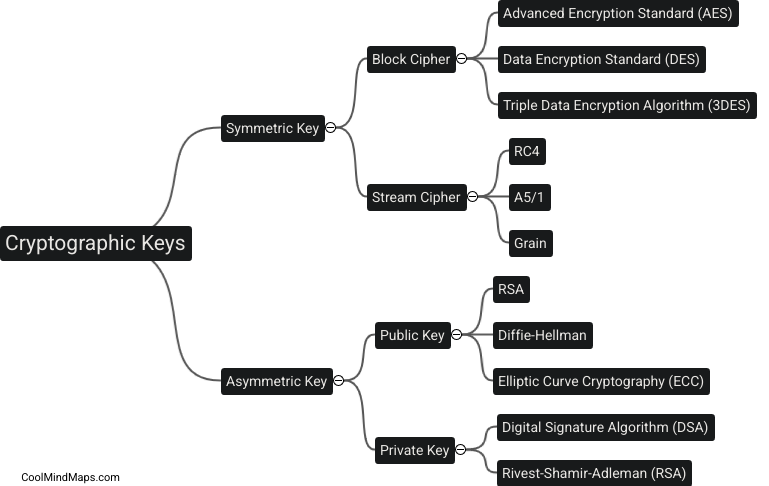
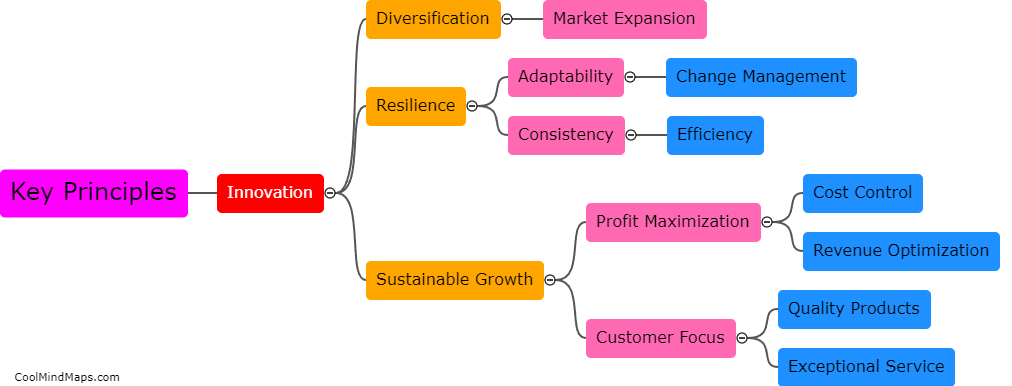
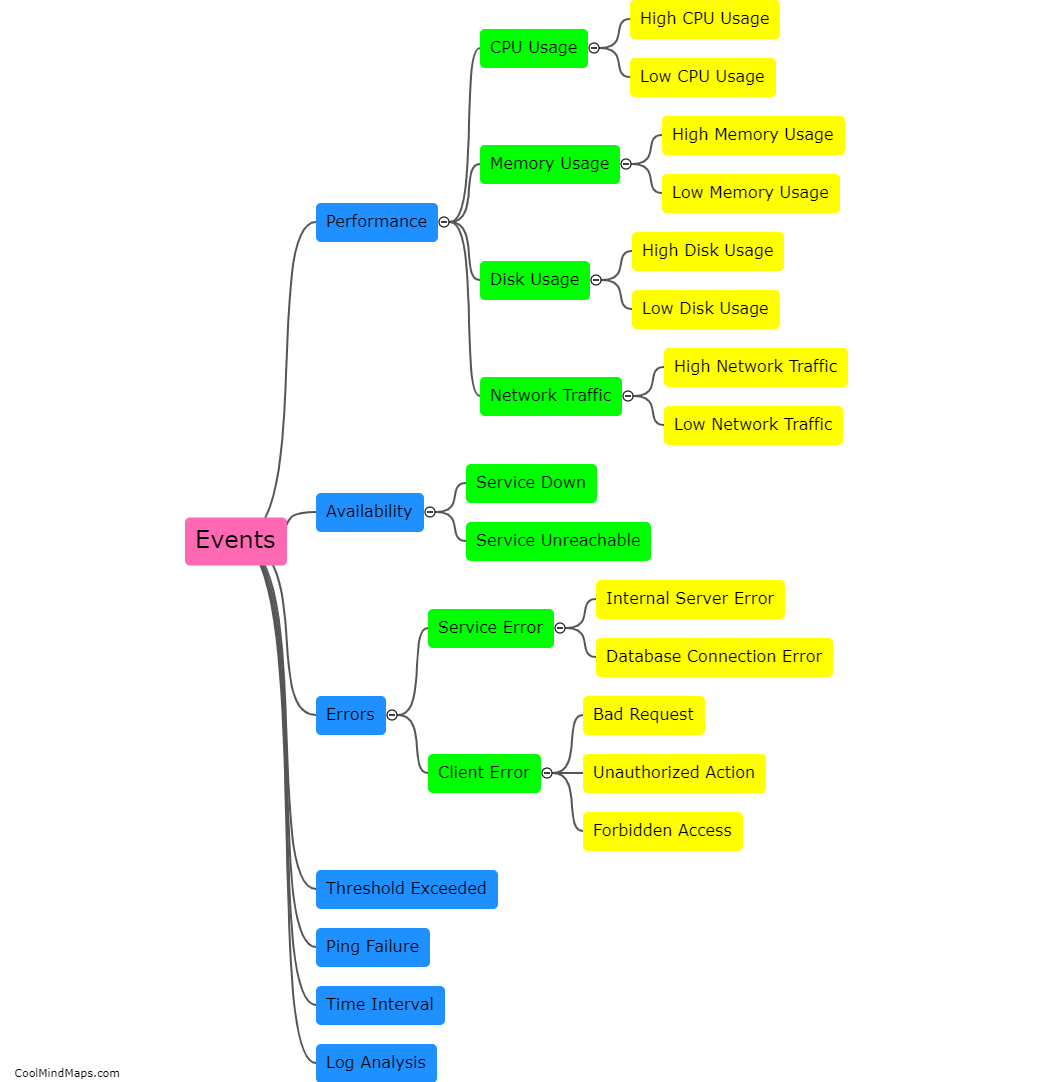
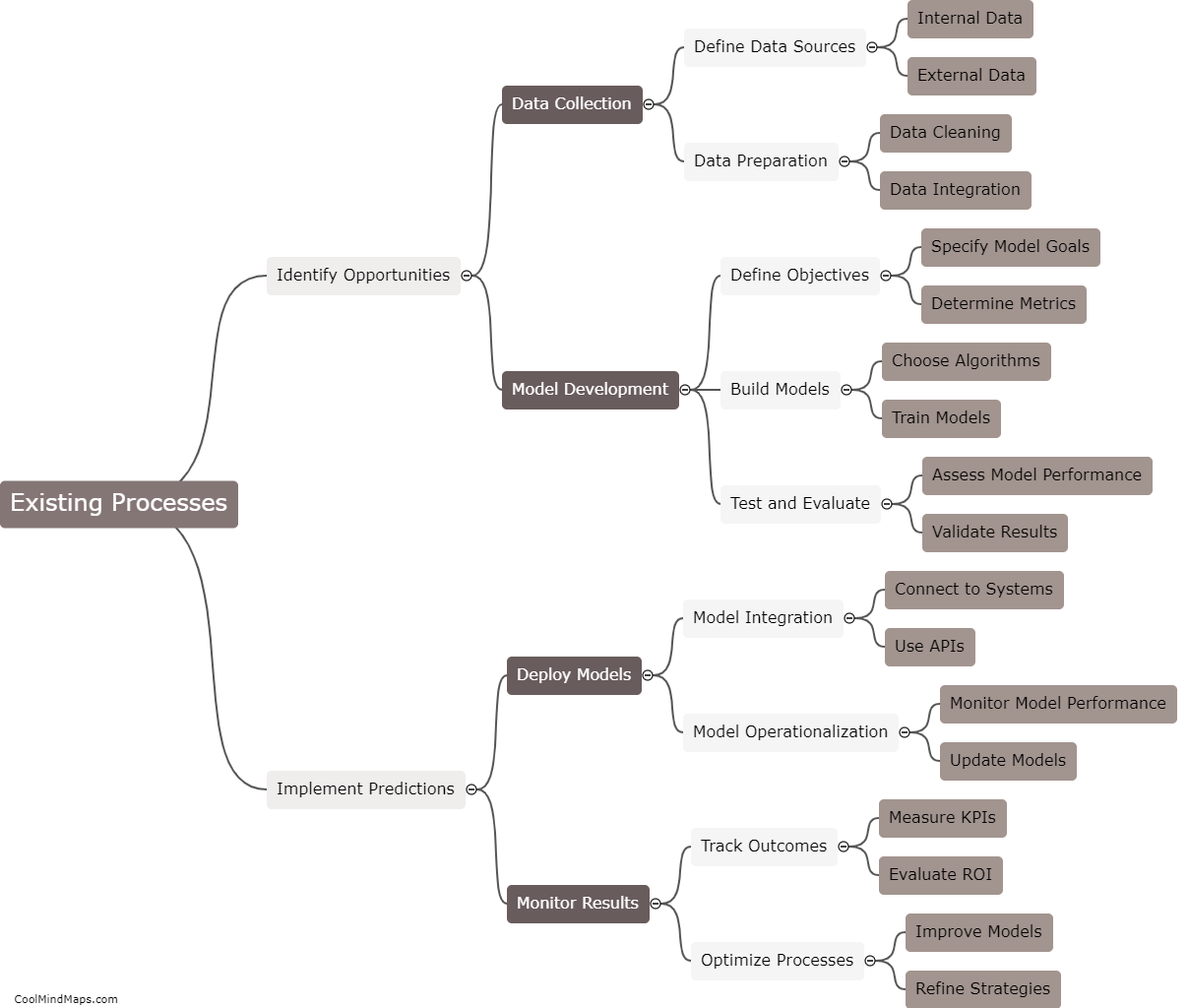
What are the key features of the atomic model?
The key features of the atomic model include the following: first, atoms are the fundamental building blocks of matter, and they cannot be divided into smaller particles. Second, atoms have a central nucleus containing protons and neutrons, with electrons orbiting the nucleus in specific energy levels or shells. Third, each element is characterized by the number of protons in the nucleus, which determines its unique atomic number. Fourth, atoms can gain or lose electrons to form ions, resulting in changes in their overall charge. Lastly, atoms of different elements can combine to form compounds through chemical reactions, where atoms are rearranged but not created or destroyed. These key features provide a basic understanding of the structure and behavior of atoms, which underpin many scientific concepts and applications in various fields.

This mind map was published on 30 July 2023 and has been viewed 124 times.