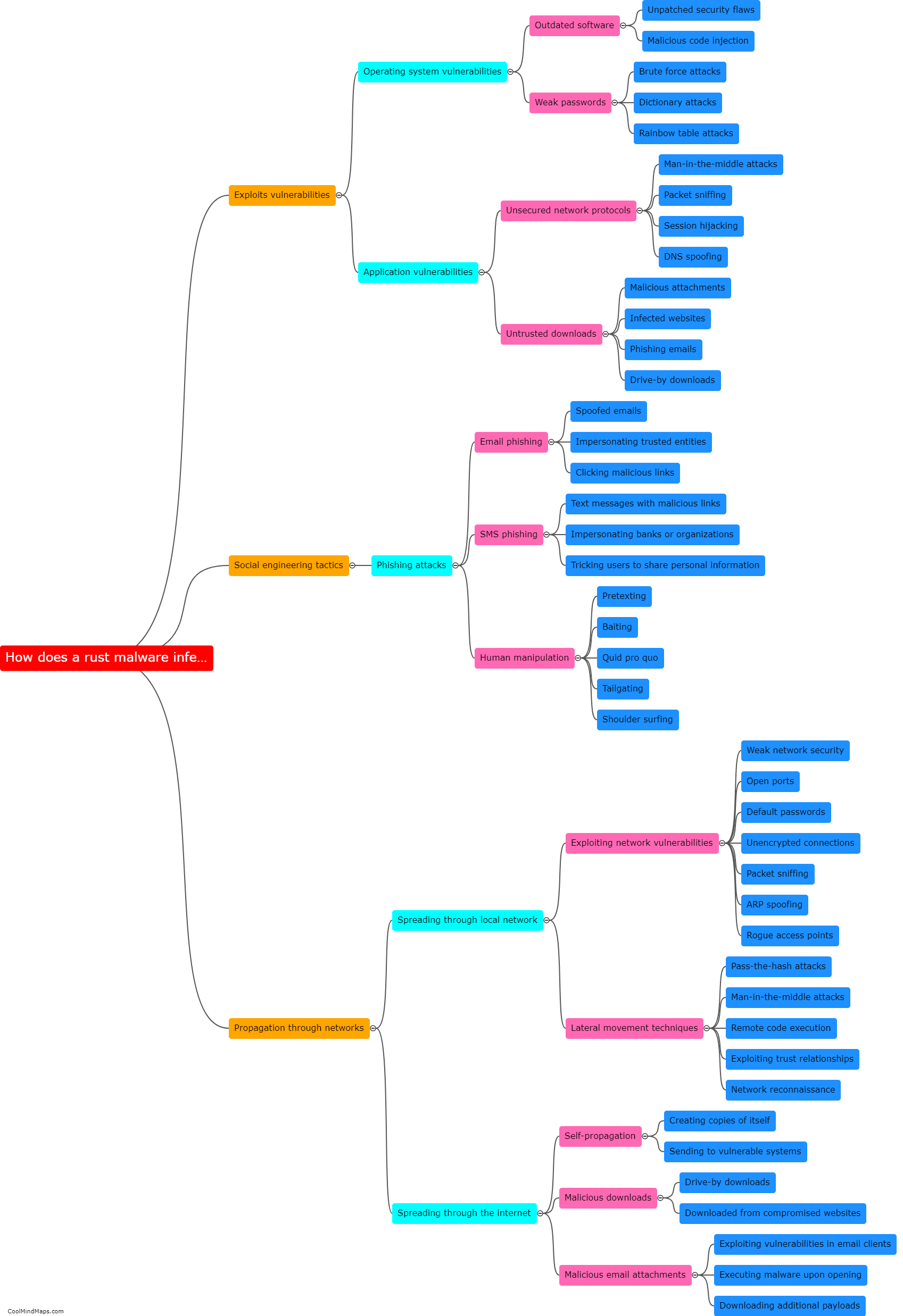
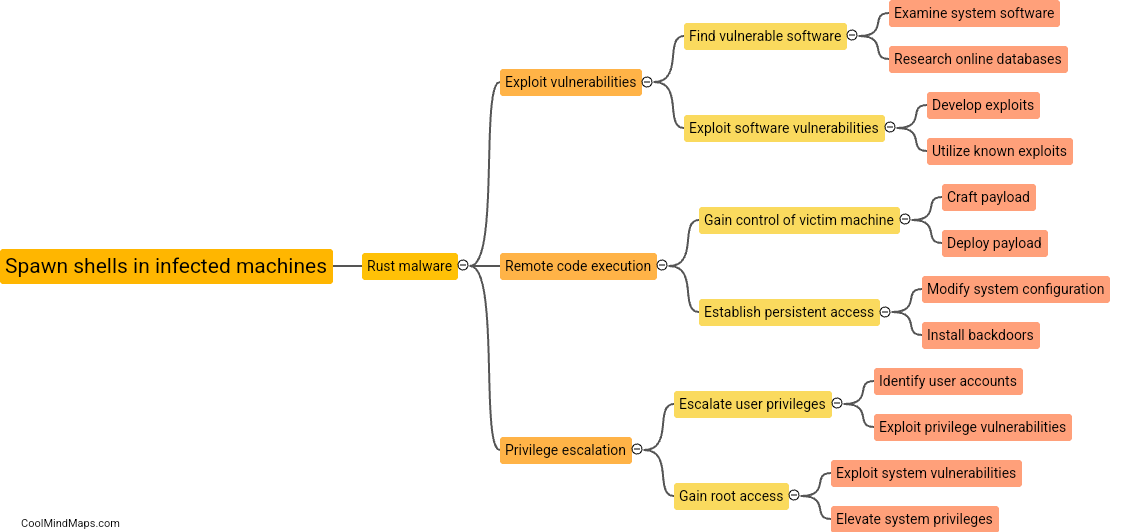
What role does the rust agent play in the process?
The rust agent, also known as iron oxide, plays a crucial role in the process of rust formation. Rusting occurs when iron or steel comes into contact with oxygen and water, leading to the formation of iron oxide. The rust agent acts as a catalyst by accelerating the oxidation reaction. It allows oxygen molecules to adhere to the metal's surface, creating a layer of iron oxide. This layer weakens the structure of the metal, leading to a degradation of its integrity over time. Therefore, the rust agent plays a significant role in the corrosion process, contributing to the deterioration of iron and steel objects.

This mind map was published on 26 October 2023 and has been viewed 84 times.