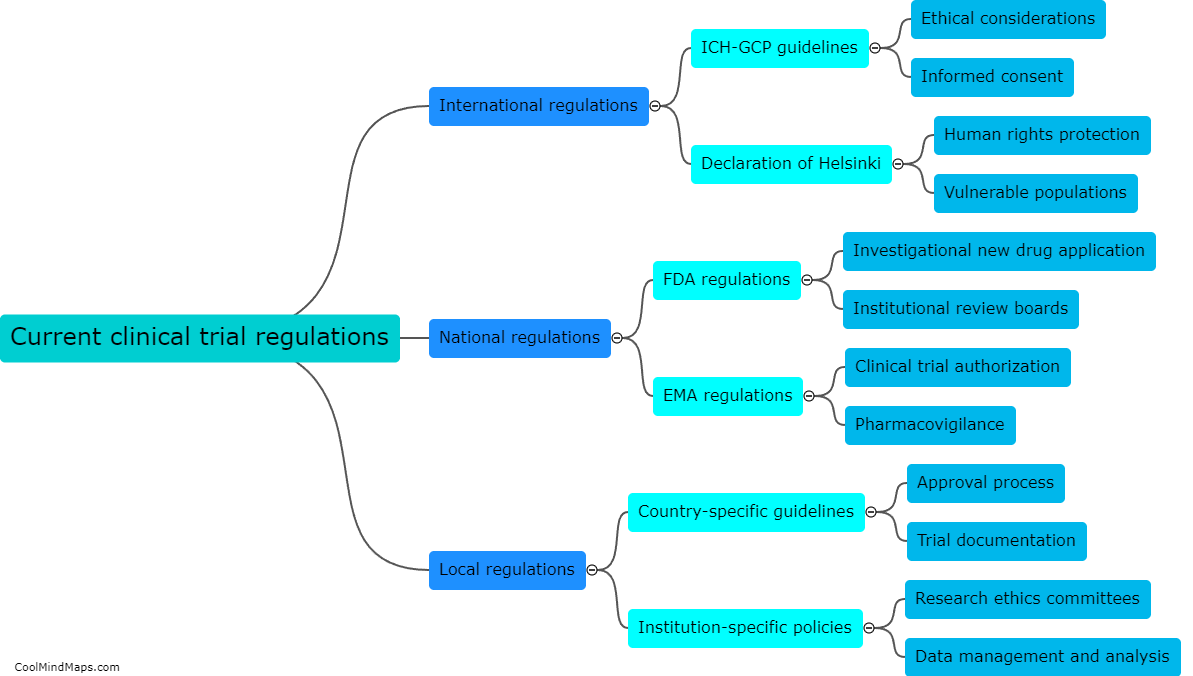
What are the current clinical trial regulations?
Clinical trial regulations are guidelines and laws put in place to ensure the ethical standards, safety, and integrity of clinical trials. These regulations vary across different countries and regions but generally involve principles like obtaining informed consent from participants, protecting their rights and welfare, minimizing risks, and transparent reporting of results. In the United States, clinical trials are regulated by the Food and Drug Administration (FDA), which enforces strict guidelines on study design, participant recruitment, data collection, and adverse event monitoring. Similar regulatory bodies exist in other countries, including the European Medicines Agency (EMA) in the European Union. The aim of these regulations is to facilitate the development of safe and effective therapies while ensuring the protection of human subjects involved in clinical trials.
This mind map was published on 7 January 2024 and has been viewed 78 times.