How do updated essential documentation methods improve the accuracy of clinical trial data?
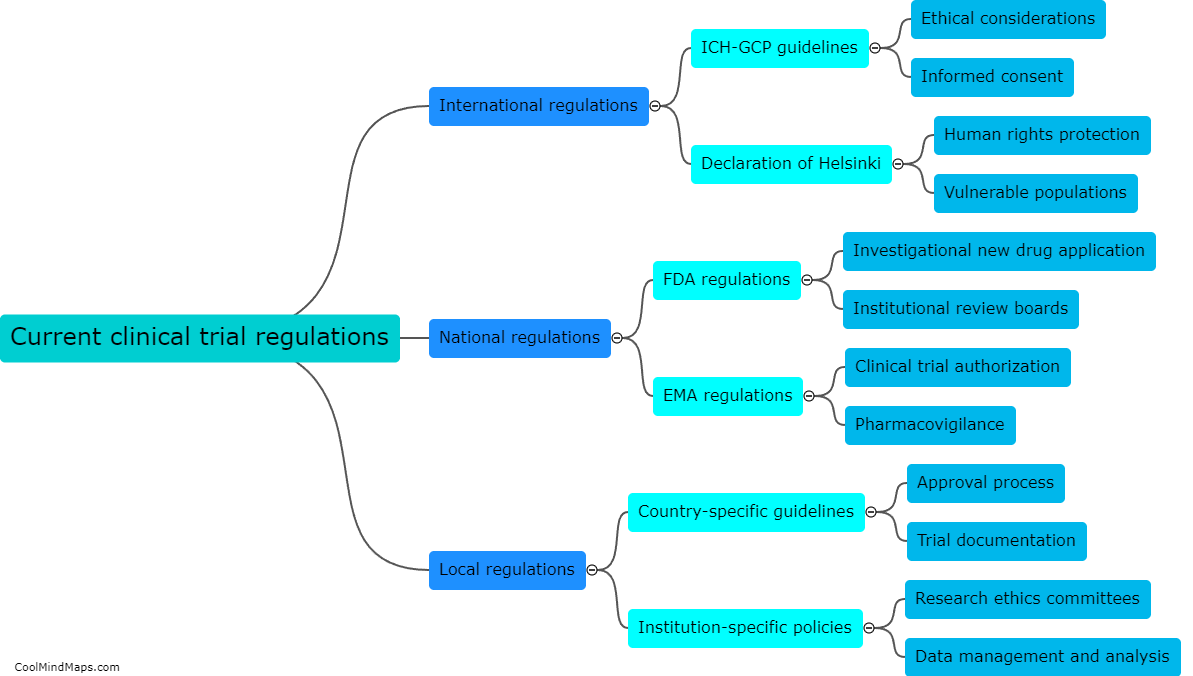
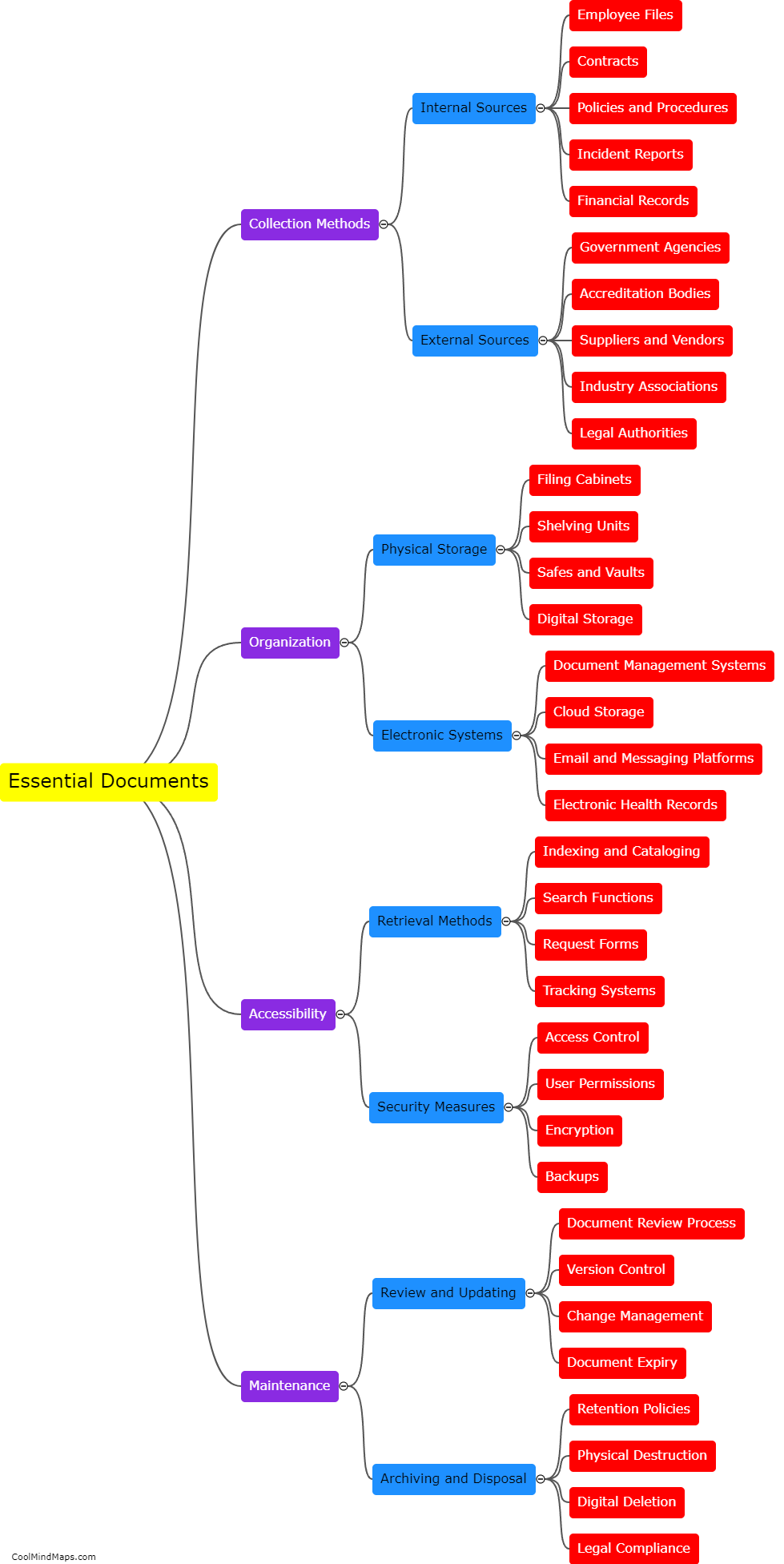
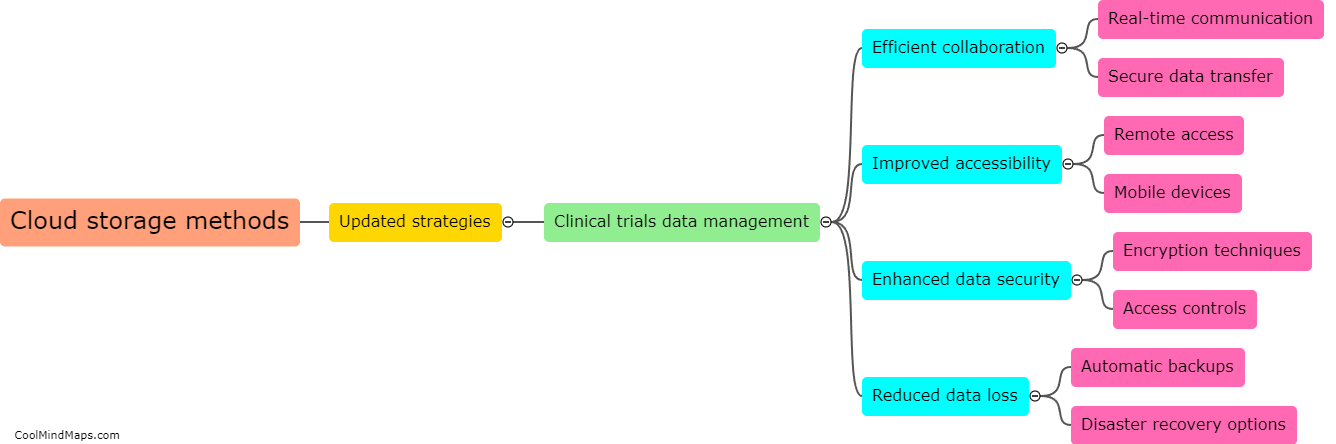
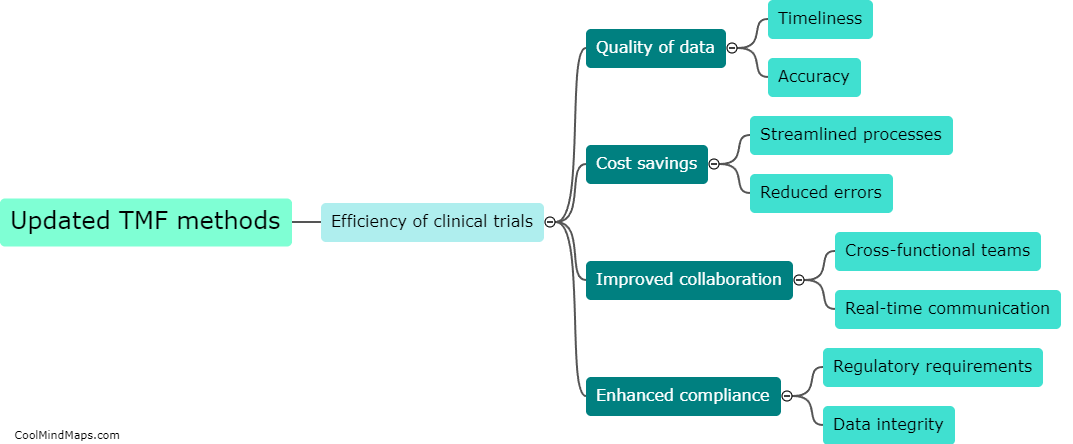
Updated essential documentation methods play a crucial role in improving the accuracy of clinical trial data. These methods ensure that all necessary documents, such as consent forms, protocols, and case report forms, are kept up to date and properly organized. By implementing standardized processes for document control and versioning, errors and discrepancies can be minimized. This ensures that all data collected during the clinical trial is accurate, reliable, and consistent. Additionally, updated documentation methods facilitate effective communication among various stakeholders involved in the trial, allowing for timely identification and resolution of any issues. Overall, these methods help to enhance the quality and integrity of clinical trial data, ensuring its validity and usefulness in advancing medical knowledge and improving patient care.
This mind map was published on 7 January 2024 and has been viewed 88 times.