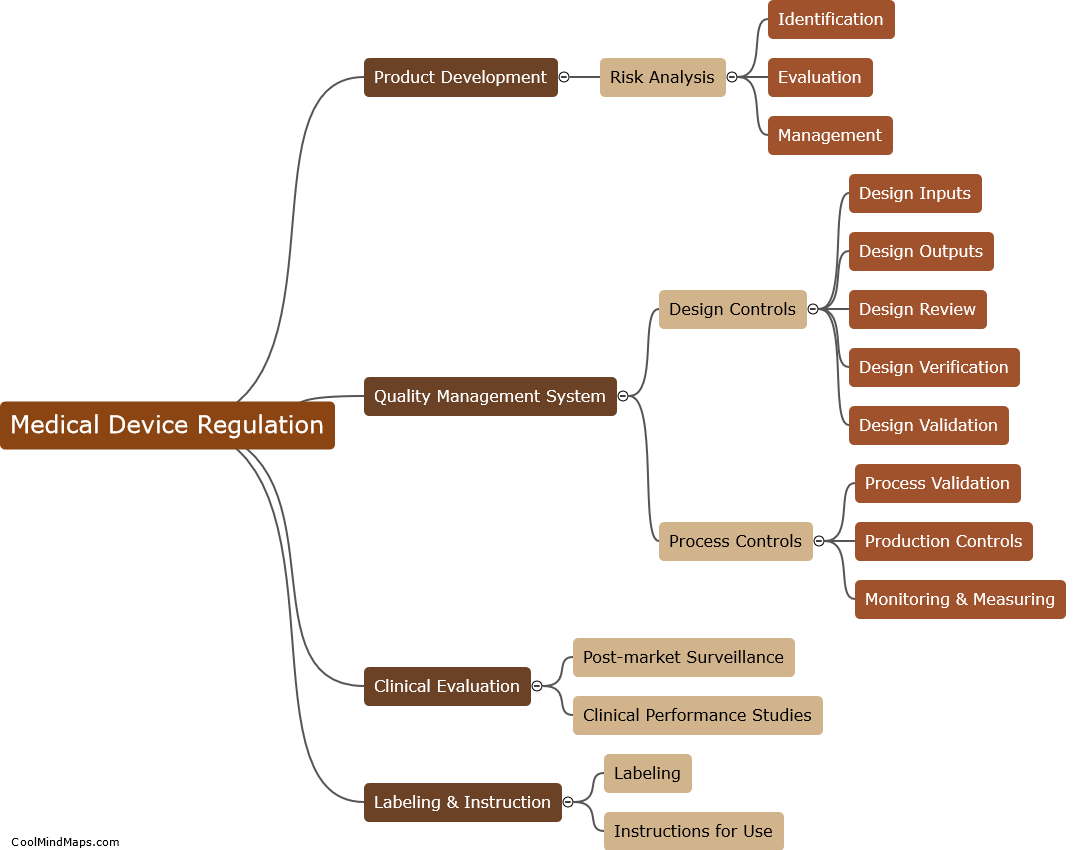
What are the key requirements of Medical Device Regulation?
The Medical Device Regulation (MDR) is a set of legal requirements that must be met by all medical devices sold in the European Union (EU). The key requirements of MDR include: compliance with safety and performance requirements, conformity assessment procedures, and post-market surveillance. Manufacturers must ensure that their devices are safe and effective and must provide appropriate documentation to demonstrate conformity with the regulation. They must also appoint a person responsible for regulatory compliance and provide clinical evidence of the device's safety and effectiveness. The regulation also mandates that devices be labeled with essential information and undergo strict post-market surveillance to ensure their ongoing safety and efficacy. Compliance with MDR is required for all medical devices sold in the EU.
This mind map was published on 23 May 2023 and has been viewed 102 times.