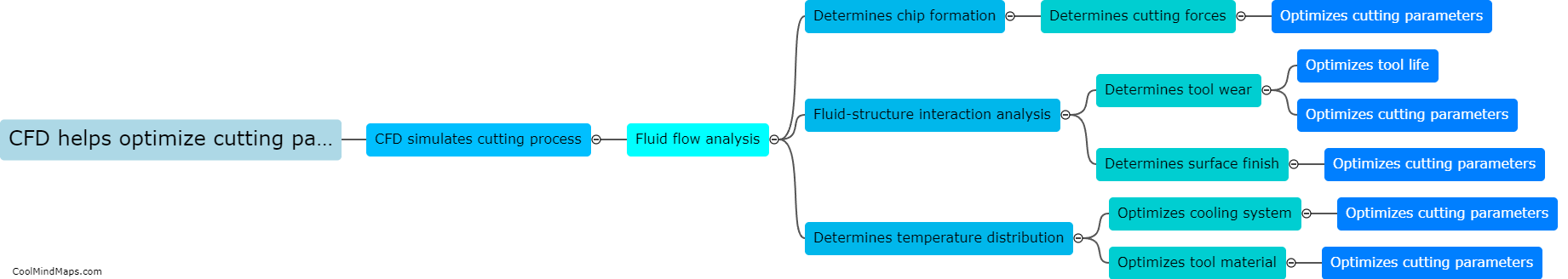
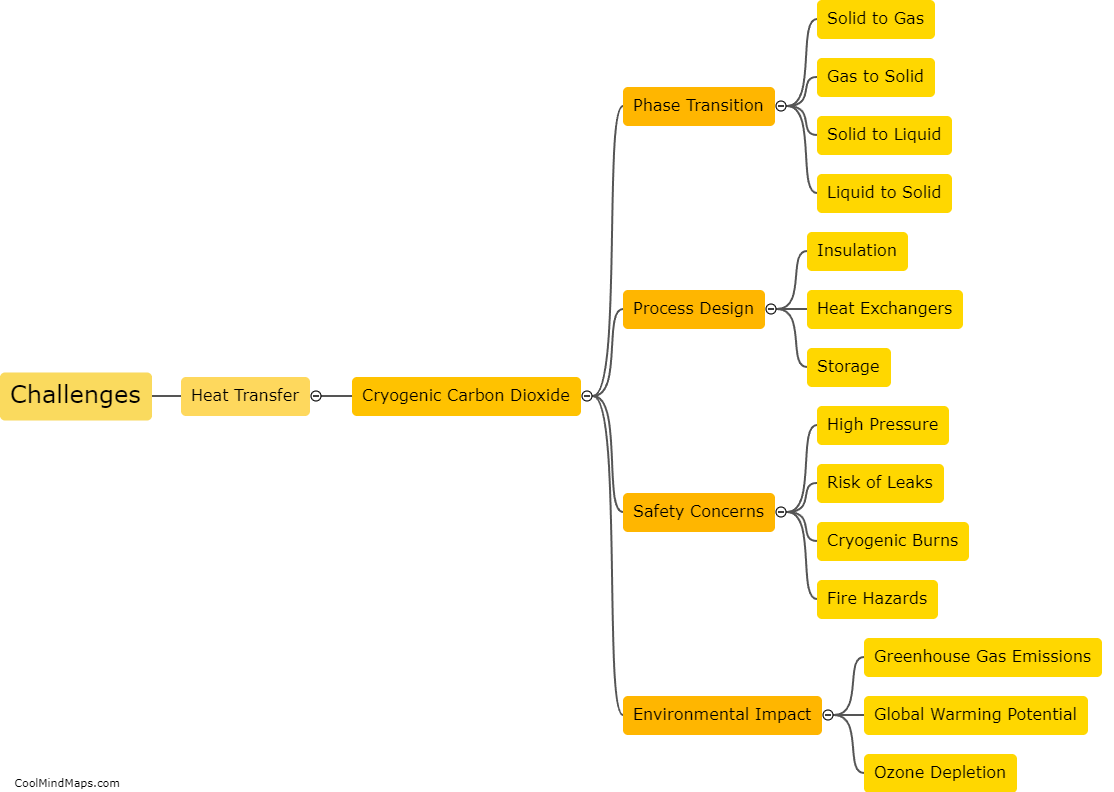
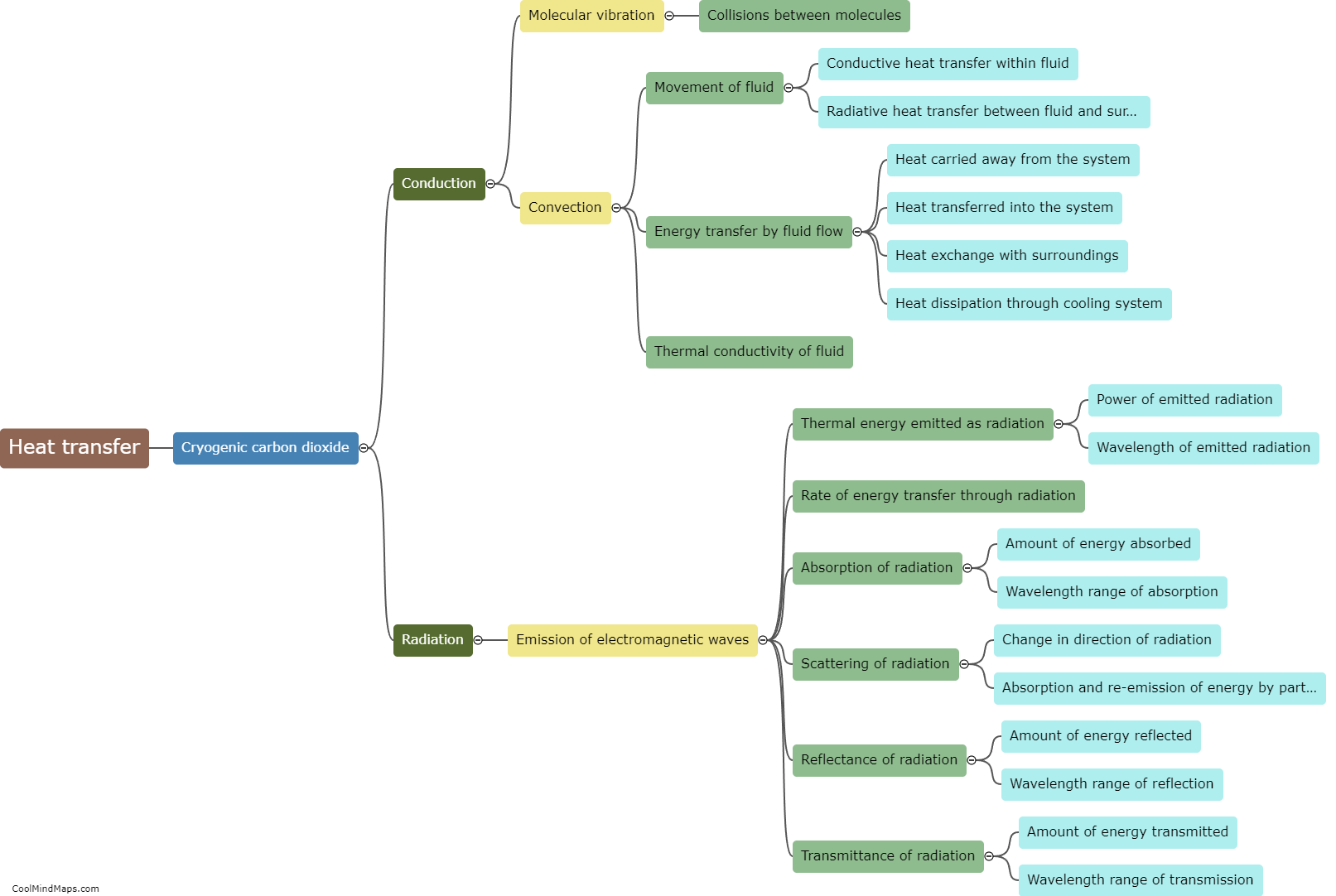
How does heat transfer occur in cryogenic carbon dioxide?
In cryogenic carbon dioxide, heat transfer occurs primarily through three mechanisms: conduction, convection, and radiation. Conduction involves the transfer of heat from one molecule to another through direct contact. In cryogenic carbon dioxide, which is stored at extremely low temperatures (-78.5°C / -109.3°F), heat transfers through the carbon dioxide molecules as they collide and exchange energy with each other. Convection, on the other hand, involves the transfer of heat through the movement of a fluid. In the case of cryogenic carbon dioxide, convective heat transfer occurs when the carbon dioxide gas moves through pipes or channels, carrying heat away from a warmer region to a cooler region. Lastly, radiation is the transfer of heat through electromagnetic waves, where energy is emitted and absorbed by objects without direct contact. In cryogenic carbon dioxide, radiation heat transfer may occur between the carbon dioxide and surrounding objects or any sources of heat nearby. Overall, these three mechanisms collectively account for heat transfer in cryogenic carbon dioxide.
This mind map was published on 30 December 2023 and has been viewed 89 times.