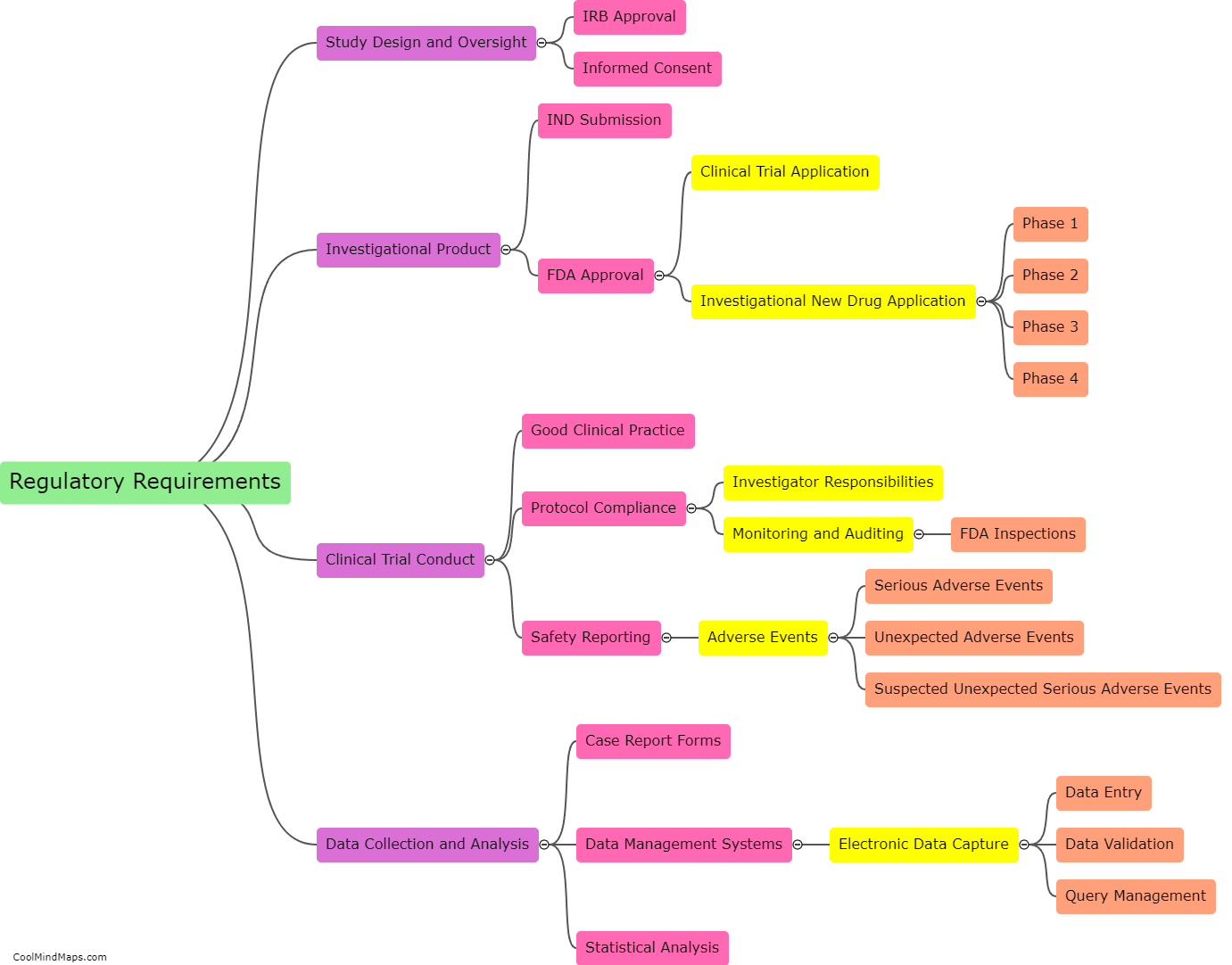
What are the regulatory requirements for a clinical trial in the US?
In the United States, conducting a clinical trial requires adherence to specific regulatory requirements aimed at protecting the rights and safety of participants. The primary regulatory body overseeing clinical trials in the US is the Food and Drug Administration (FDA). The key requirements include the submission of an Investigational New Drug (IND) application for investigational drugs or a Investigational Device Exemption (IDE) for investigational medical devices. These applications include comprehensive information on the drug or device, its manufacturing, preclinical data, and the proposed clinical trial protocol. Additionally, the trial must undergo review and approval by an Institutional Review Board (IRB), which ensures ethical standards are met. Throughout the trial, sponsors must adhere to Good Clinical Practice (GCP) guidelines, maintain accurate records, report adverse events, and comply with FDA inspections. These regulatory requirements aim to ensure the quality, integrity, and safety of clinical research in the US.
This mind map was published on 23 July 2023 and has been viewed 154 times.