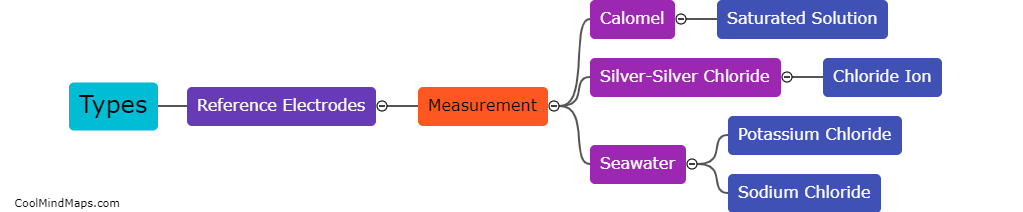
What are the types of reference electrodes?
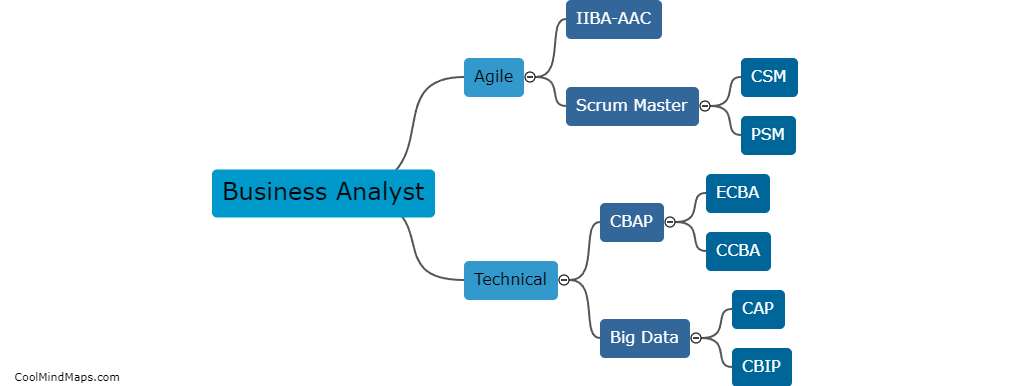
Reference electrodes are an essential component in electrochemical measurements and experiments. These electrodes provide a stable voltage reference against which the potential of the working electrode can be measured accurately. There are several types of reference electrodes commonly used, each with its own advantages and limitations. The most widely employed reference electrode is the standard hydrogen electrode (SHE), which uses a reversible hydrogen reaction to establish a zero-voltage reference. Other types include the silver/silver chloride electrode (Ag/AgCl), which is commonly used in bioelectrochemistry due to its robustness and stability, and the calomel electrode (Hg/Hg2Cl2), which is commonly used in analytical applications. Additionally, reference electrodes can also be constructed using other materials such as platinum, lead, or saturated calomel, depending on the specific requirements of the electrochemical experiment.
This mind map was published on 14 December 2023 and has been viewed 88 times.