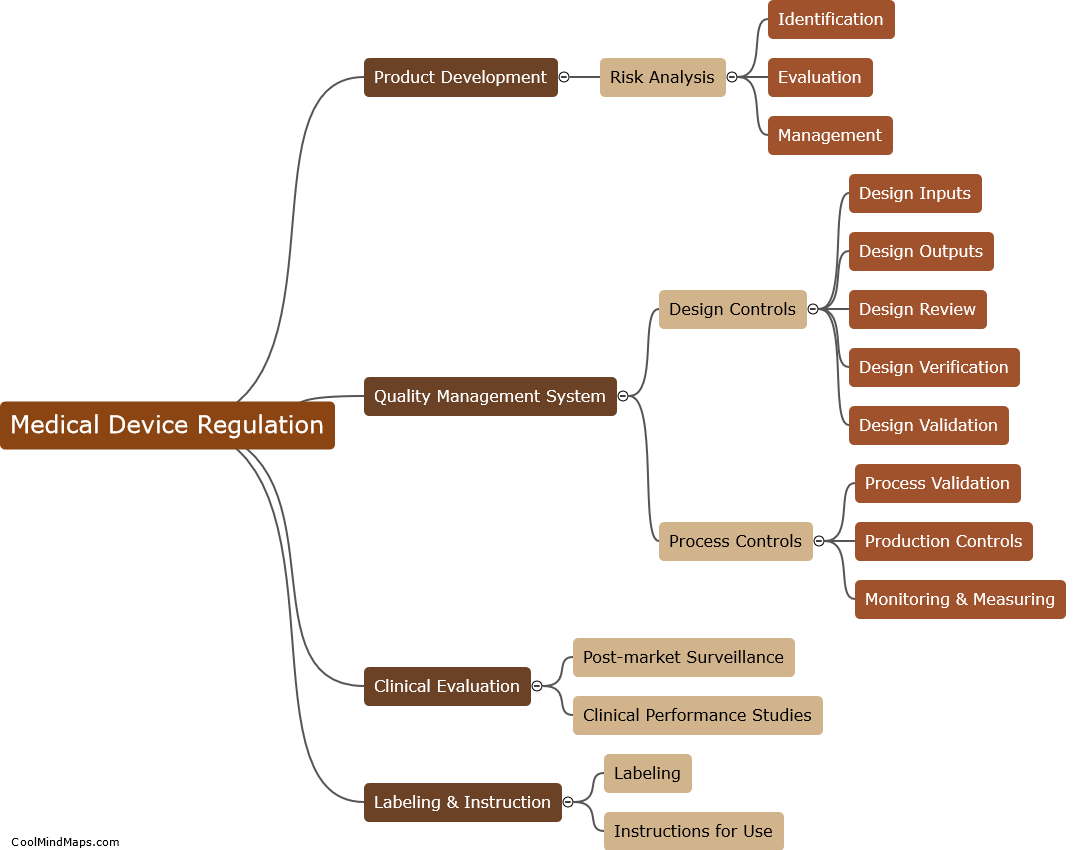
How do you ensure compliance with Medical Device Regulation?
Ensuring compliance with Medical Device Regulation involves a range of activities to ensure medical devices are safe and effective for use by patients. In order to achieve compliance, medical device manufacturers must develop quality systems that ensure their devices meet the regulatory requirements set out by governing bodies such as the FDA and the MDR. These systems often involve a number of steps including testing to ensure the device is fit for purpose, documenting the manufacturing process to demonstrate quality control, and implementing a risk management process to identify and mitigate potential safety risks. By closely following these guidelines and constantly improving their processes, medical device manufacturers can ensure that their products meet the required standards and ultimately benefit the patients who rely on them.

This mind map was published on 23 May 2023 and has been viewed 111 times.