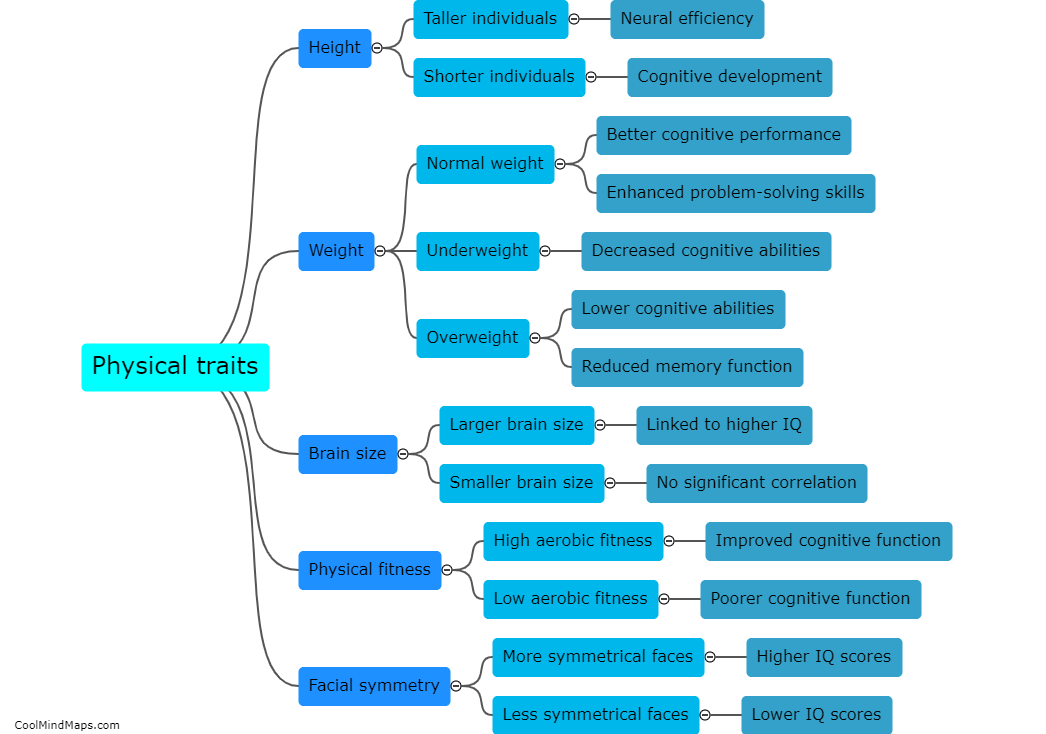
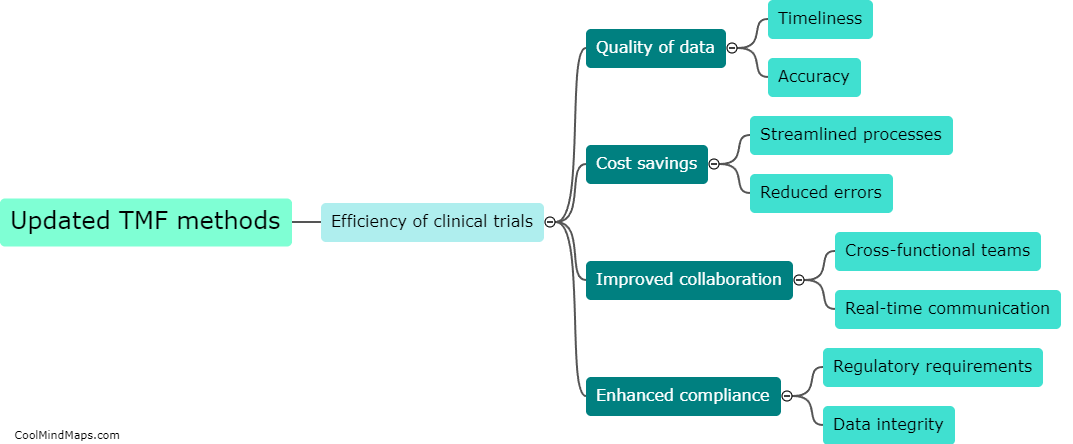
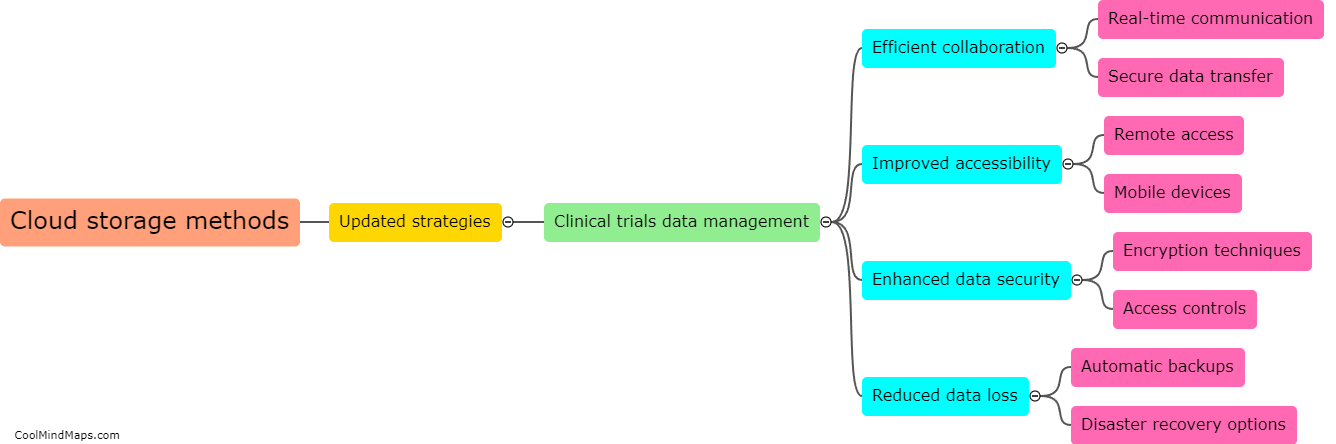
How do updated cloud storage methods improve data management in clinical trials?
Updated cloud storage methods have significantly improved data management in clinical trials. These methods provide a secure and accessible platform for storing and analyzing large volumes of clinical trial data. Cloud storage eliminates the need for physical storage space and reduces the risk of data loss or damage. With updated cloud storage methods, researchers can easily store, share, and collaborate on data remotely. This allows for real-time data access, enabling more efficient monitoring of trial progress and facilitating faster decision-making. Additionally, cloud storage offers advanced data encryption and security measures, ensuring the protection of sensitive patient information. Overall, updated cloud storage methods have revolutionized data management in clinical trials, streamlining processes and improving the quality and efficiency of research.
This mind map was published on 7 January 2024 and has been viewed 88 times.