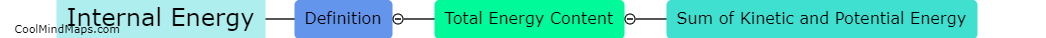How is enthalpy calculated in thermodynamic processes?
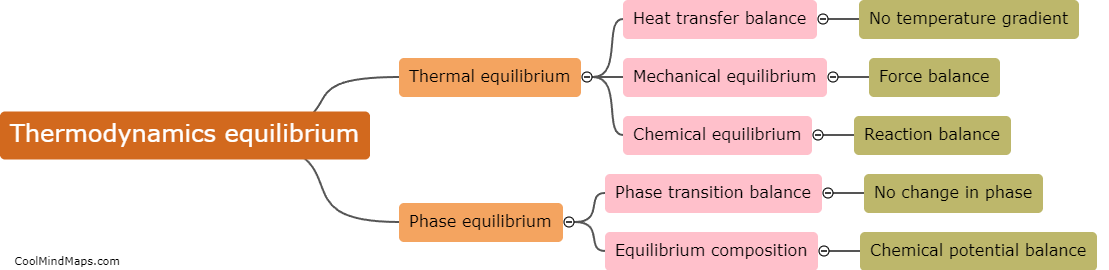
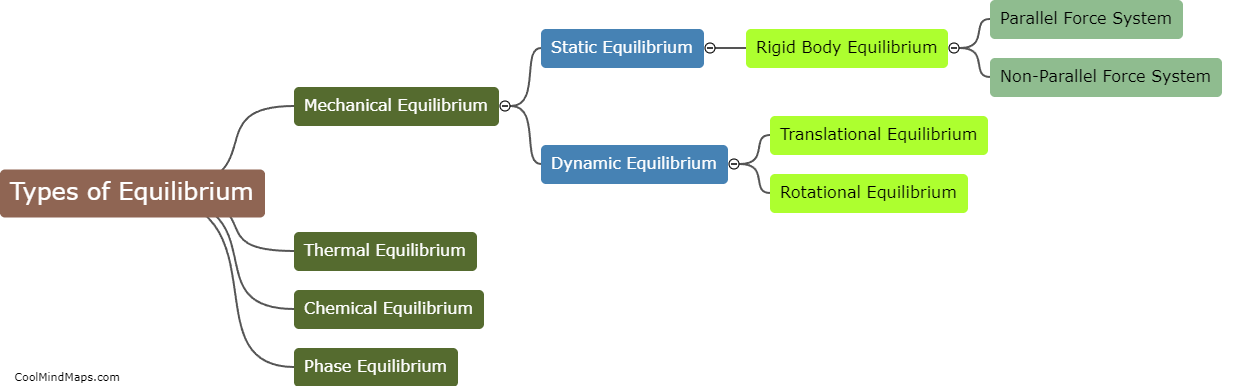
Enthalpy is a key concept in thermodynamics that measures the total heat energy content of a system. It is calculated using the principles of thermodynamics and involves determining the difference in energy between the initial and final states of a process. The calculation of enthalpy takes into account the internal energy of the system as well as any work done on or by the system. In most cases, enthalpy is calculated by measuring the heat transfer that occurs during a process, often through changes in temperature or pressure. This allows for a quantitative assessment of the energy exchange and helps in understanding the heat flow and energy changes associated with thermodynamic processes. The enthalpy calculations are crucial for analysing various engineering applications, such as power generation, heating and cooling systems, and chemical reactions.

This mind map was published on 19 September 2023 and has been viewed 89 times.