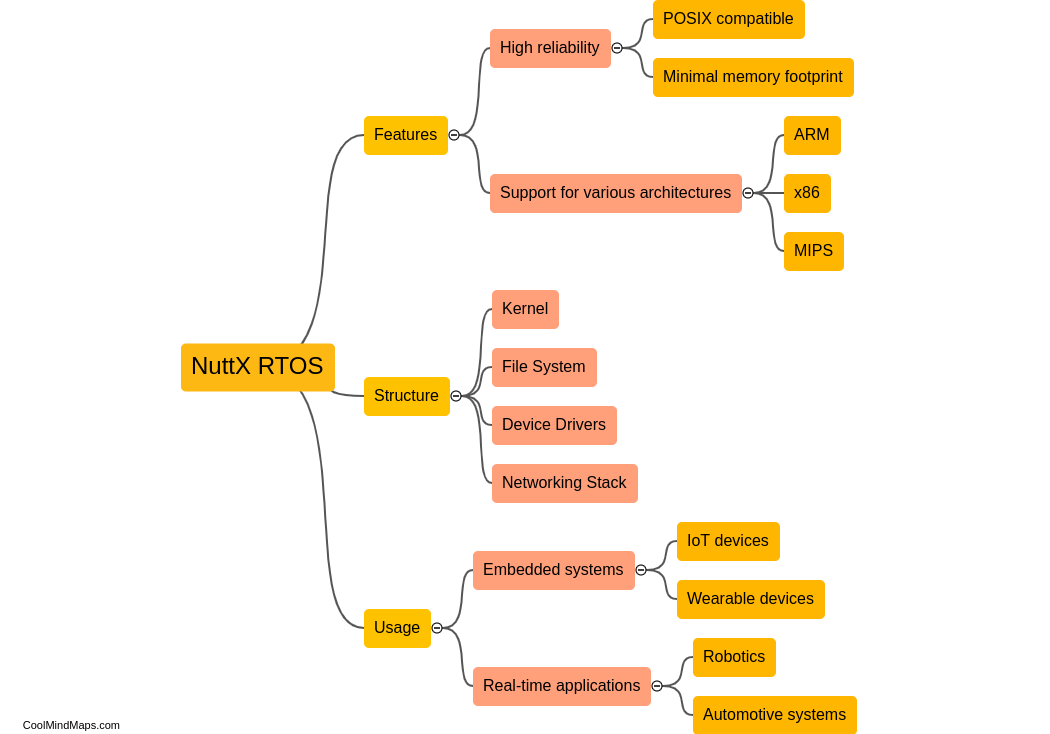
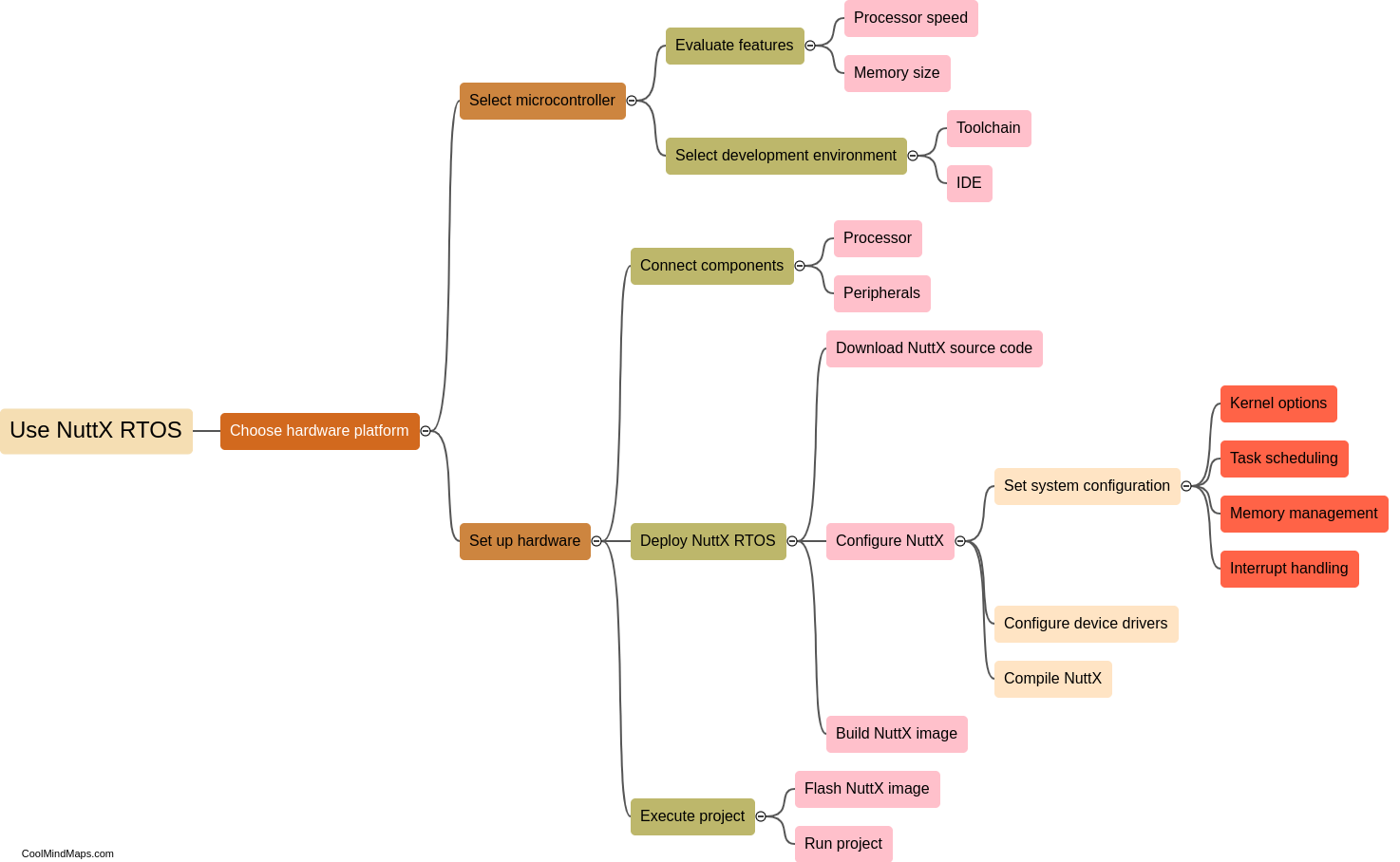
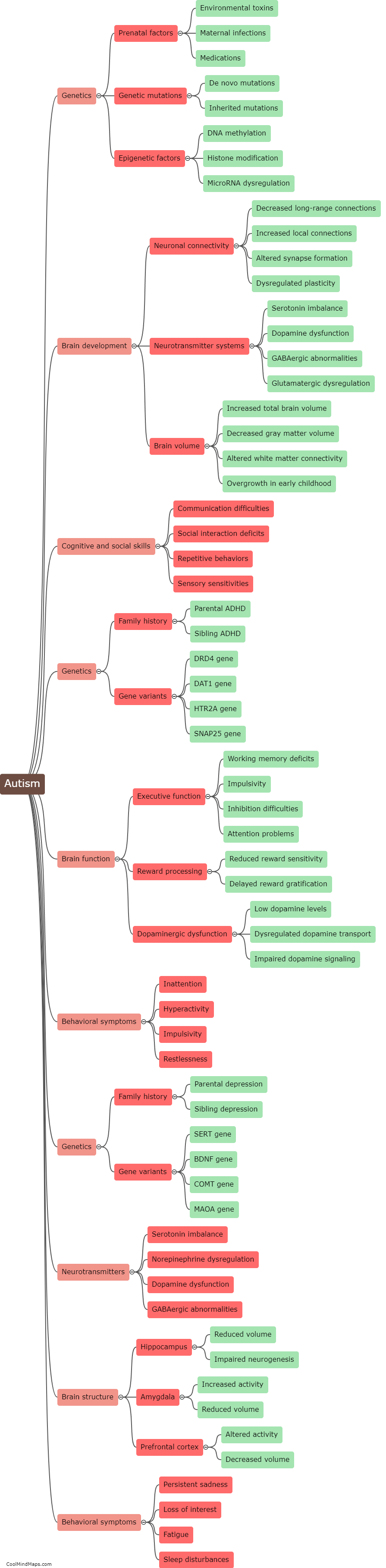
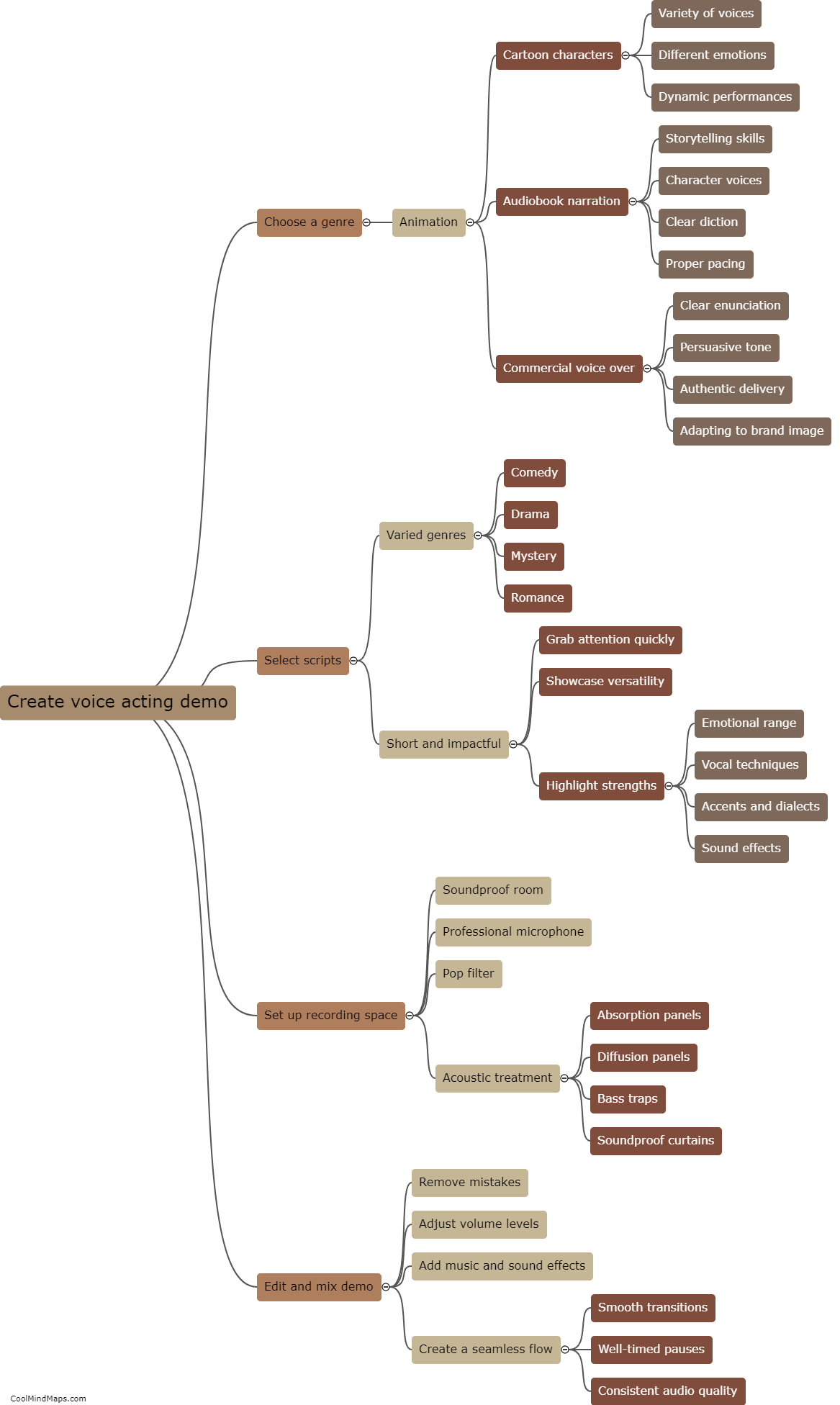
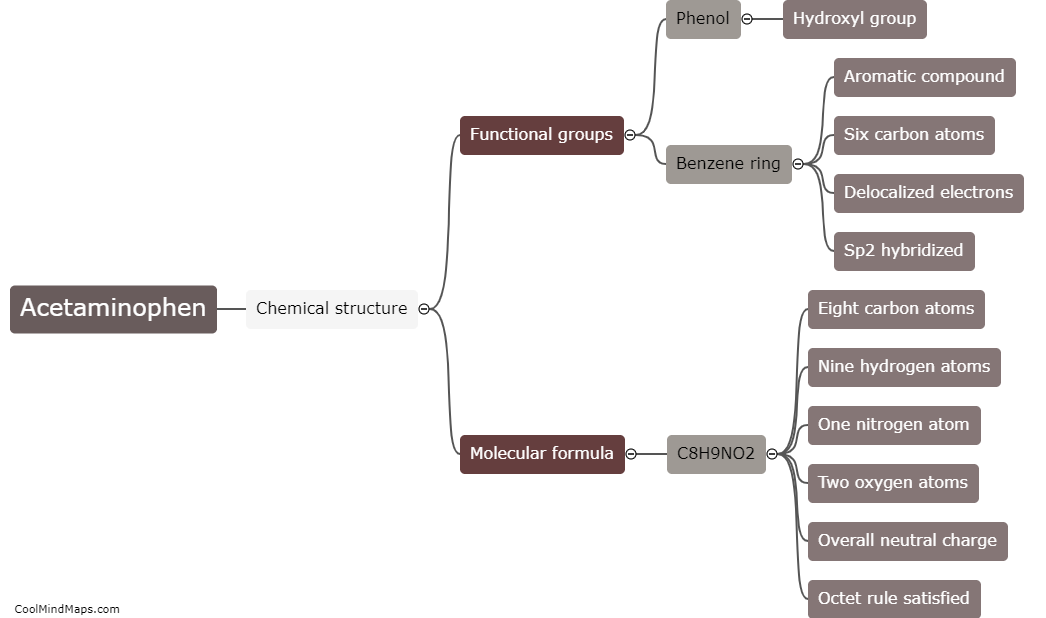
What is the chemical structure of acetaminophen?
The chemical structure of acetaminophen, also known as N-acetyl-p-aminophenol or paracetamol, consists of a benzene ring attached to an acetyl group (CH3CO-) and an amino group (NH2) at the para position. The molecular formula is C8H9NO2. The presence of the acetyl group makes acetaminophen an amide derivative of para-aminophenol. This unique structure is responsible for the compound's analgesic (pain-relieving) and antipyretic (fever-reducing) properties. Acetaminophen is a widely used over-the-counter medication for the relief of pain and fever.
This mind map was published on 9 July 2023 and has been viewed 104 times.