How should a TMF be organized?
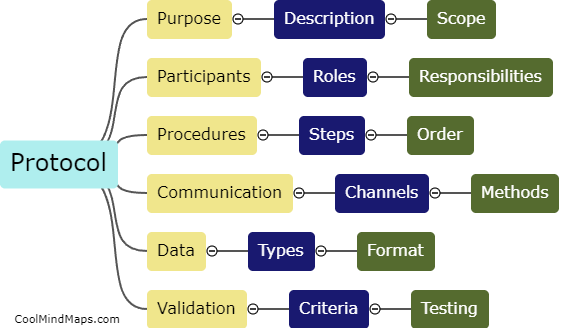
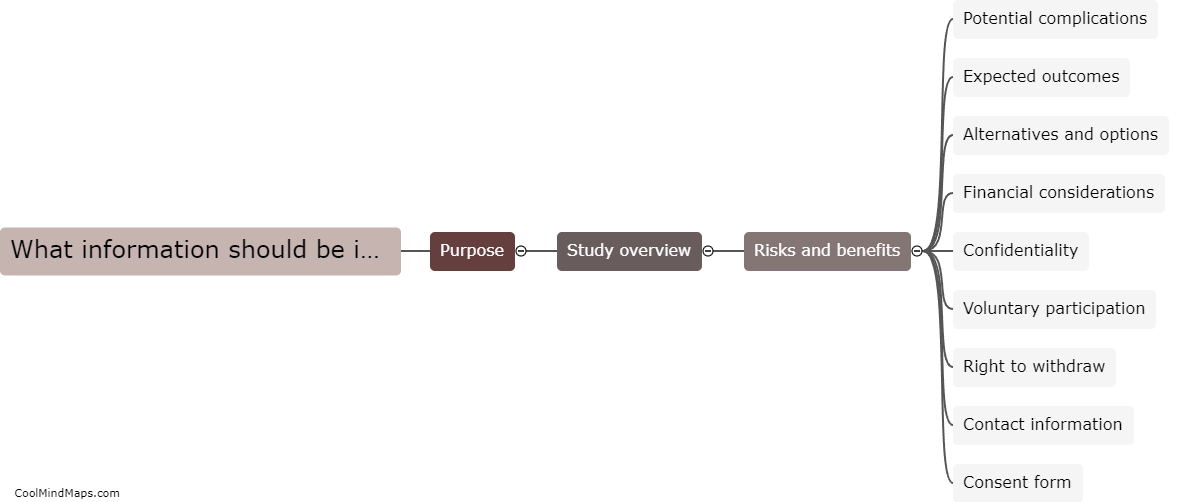
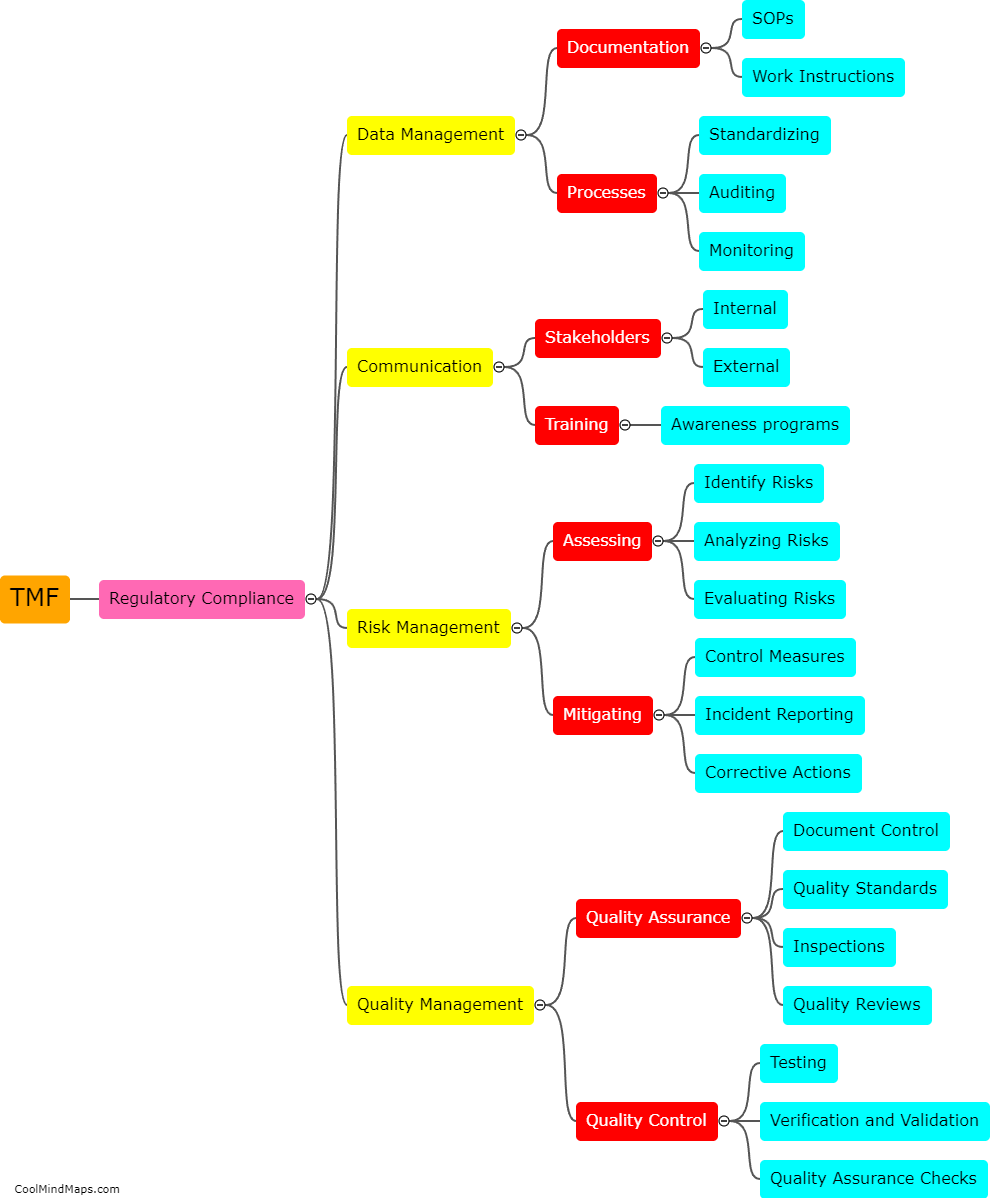
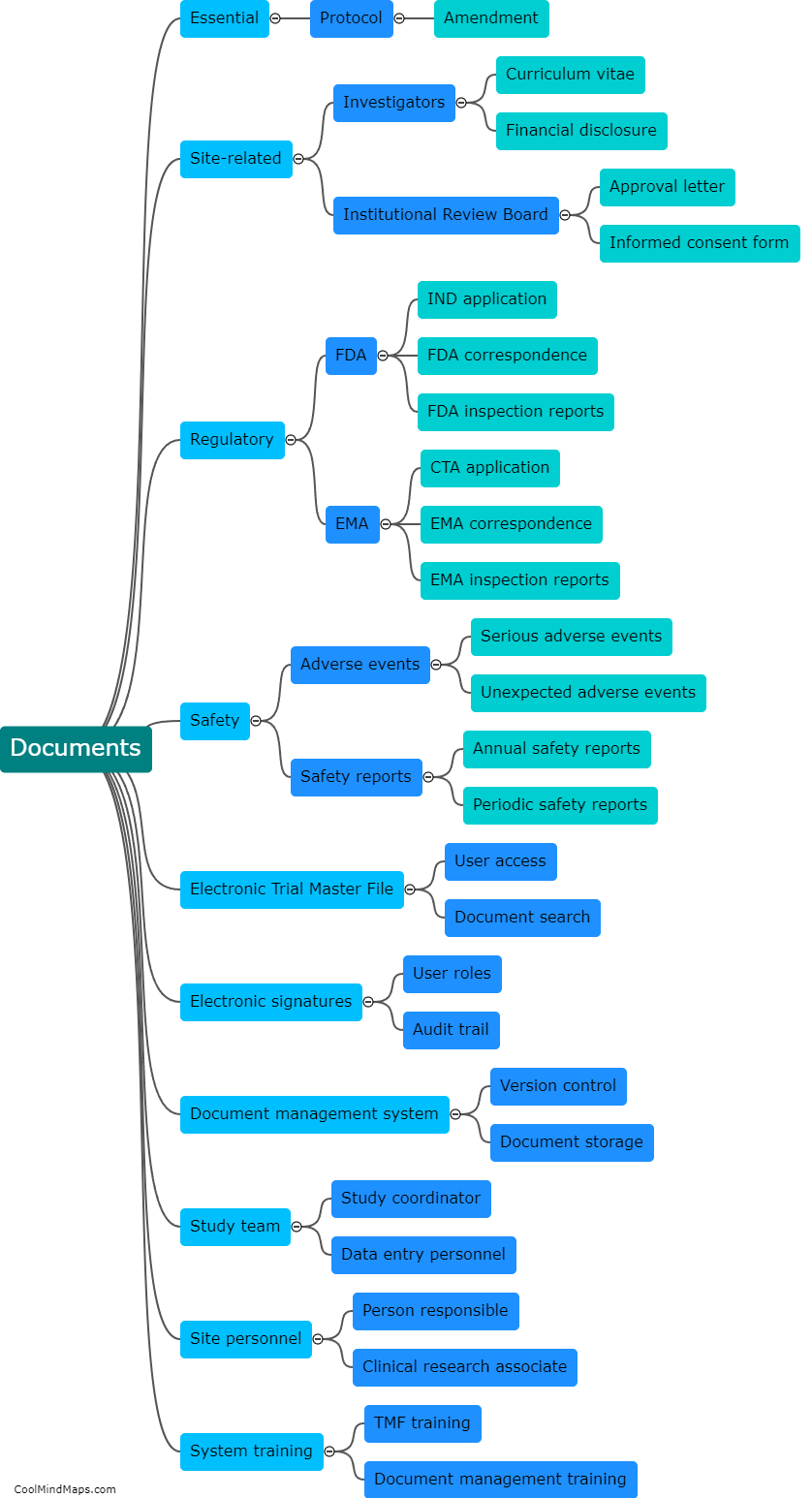
A TMF (Trial Master File) is a critical component of clinical trials, containing all the essential documents and information related to the conduct of the study. Organizing a TMF is crucial to ensure efficient and effective study management. The TMF should be organized in a systematic and logical manner, with clear and standardized processes for document filing and retrieval. It is recommended to categorize documents by their type or function, such as investigator brochures, protocol-related documents, informed consent forms, safety reports, and monitoring documentation. Each category should have a designated section within the TMF, with sub-folders or tabs for easy navigation. Maintaining an index or table of contents can further facilitate document retrieval. Additionally, implementing version control measures and ensuring consistency in document naming and formatting enhances the organization of the TMF. Regular reviews and audits should be conducted to ensure compliance with regulatory requirements and proper organization of the TMF.
This mind map was published on 5 December 2023 and has been viewed 99 times.