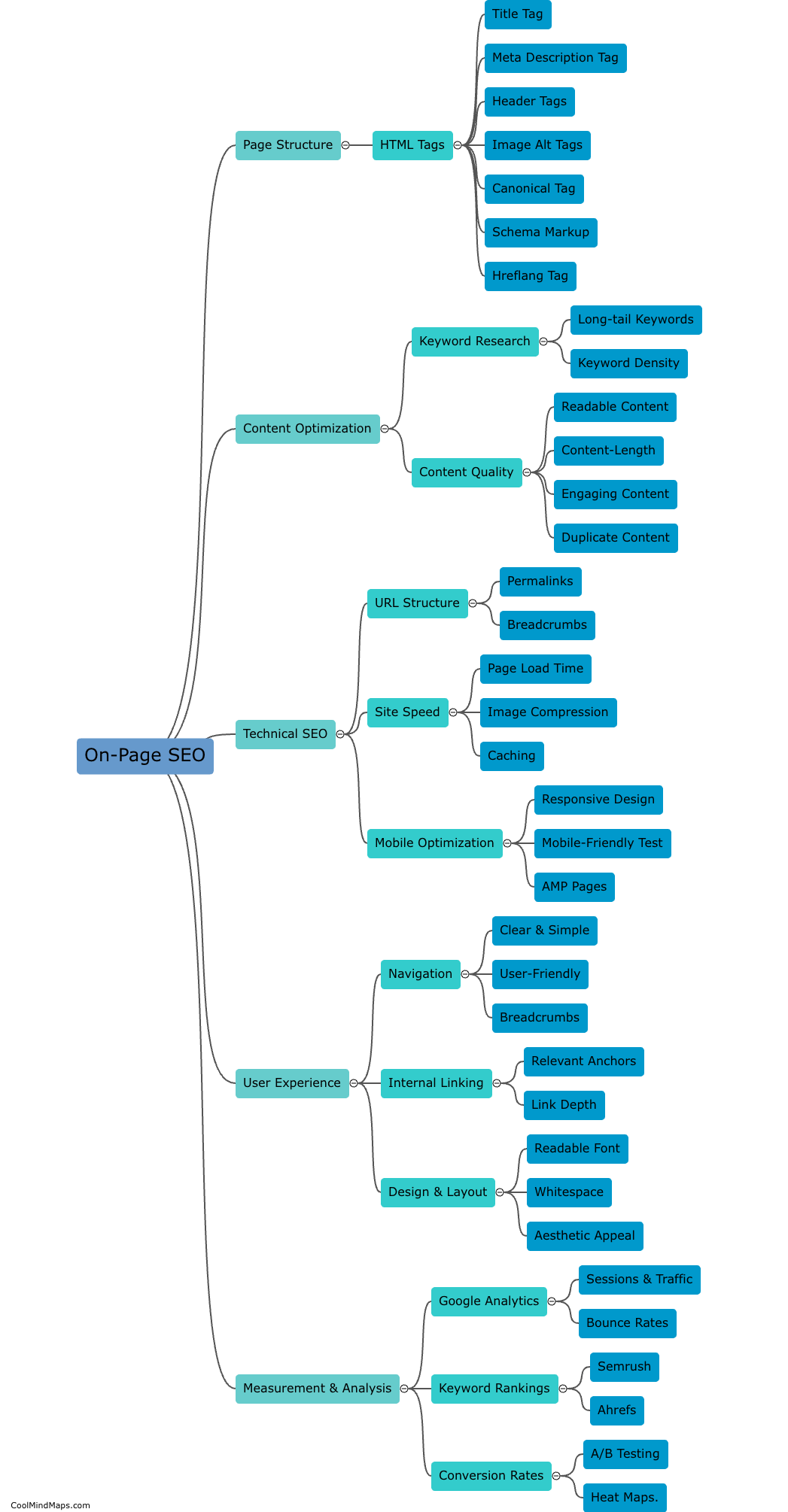
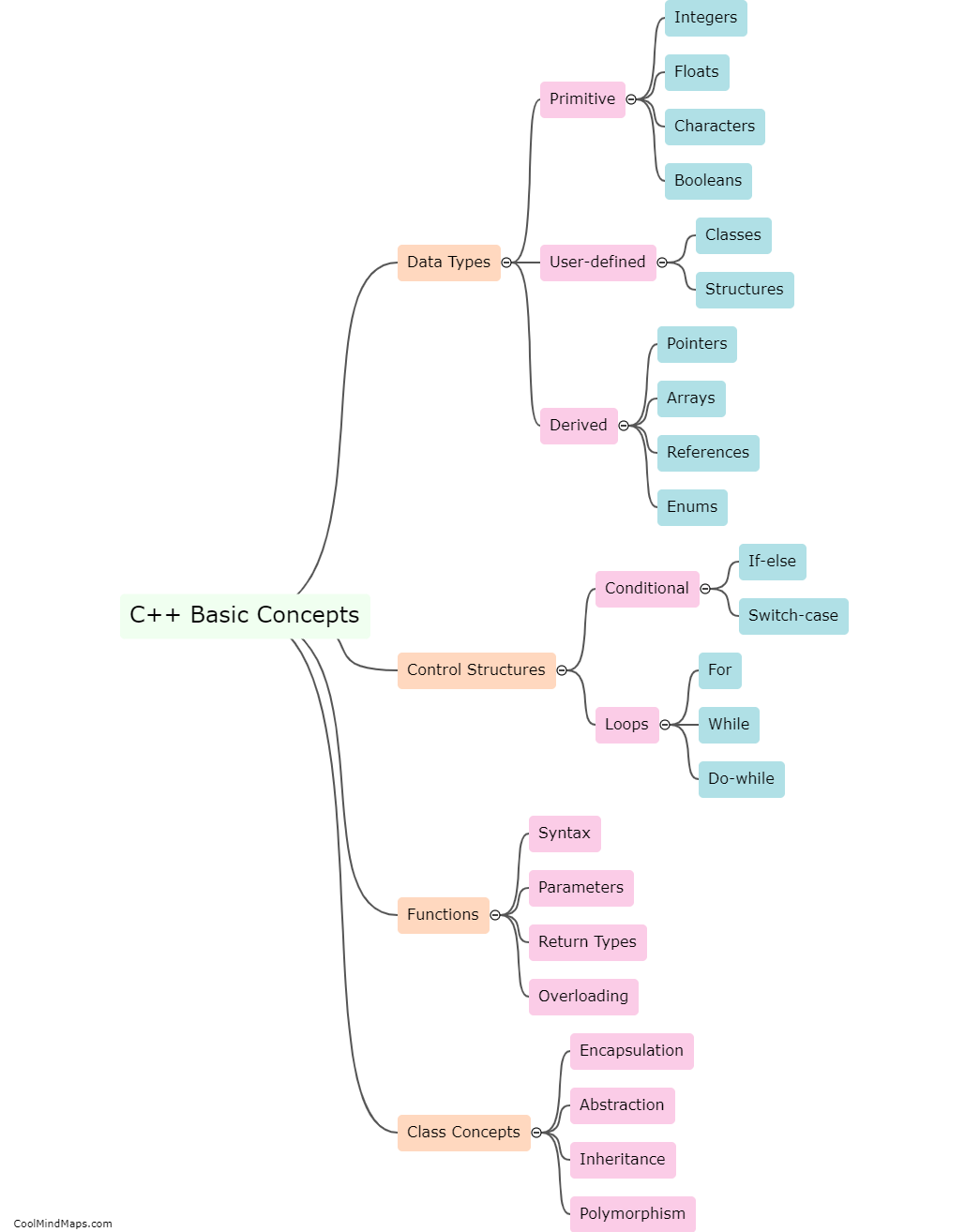
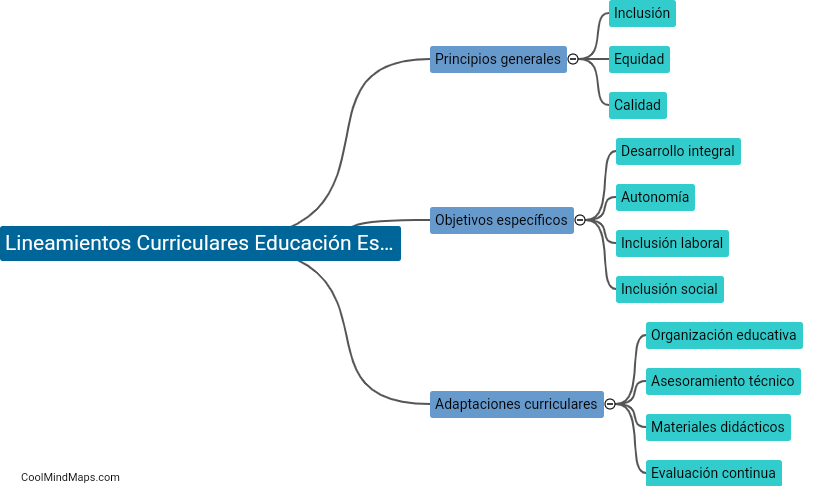
What are Faraday's laws of electrolysis?
Faraday's laws of electrolysis state that the amount of a substance deposited or produced during electrolysis is directly proportional to the amount of electric charge passed through the electrolyte. Additionally, the mass of a substance deposited is directly proportional to its atomic weight. These laws, developed by British scientist Michael Faraday, established the quantitative relationship between the amount of electricity and the chemical changes produced during electrolysis. They have been fundamental in the study and understanding of electrochemistry and its applications in various fields.

This mind map was published on 2 May 2023 and has been viewed 95 times.