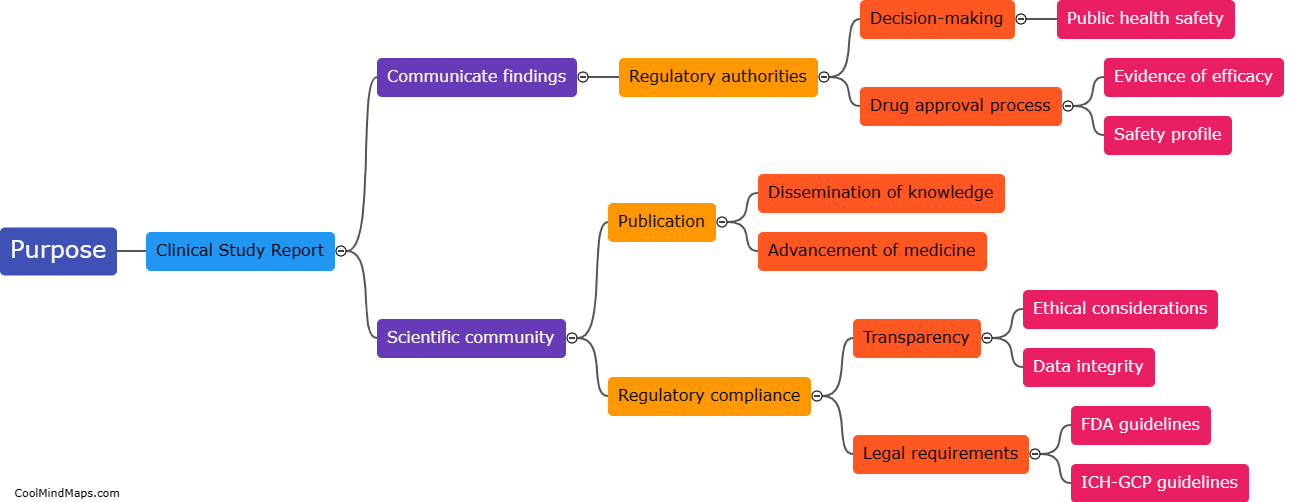
What is the purpose of a clinical study report in clinical research?
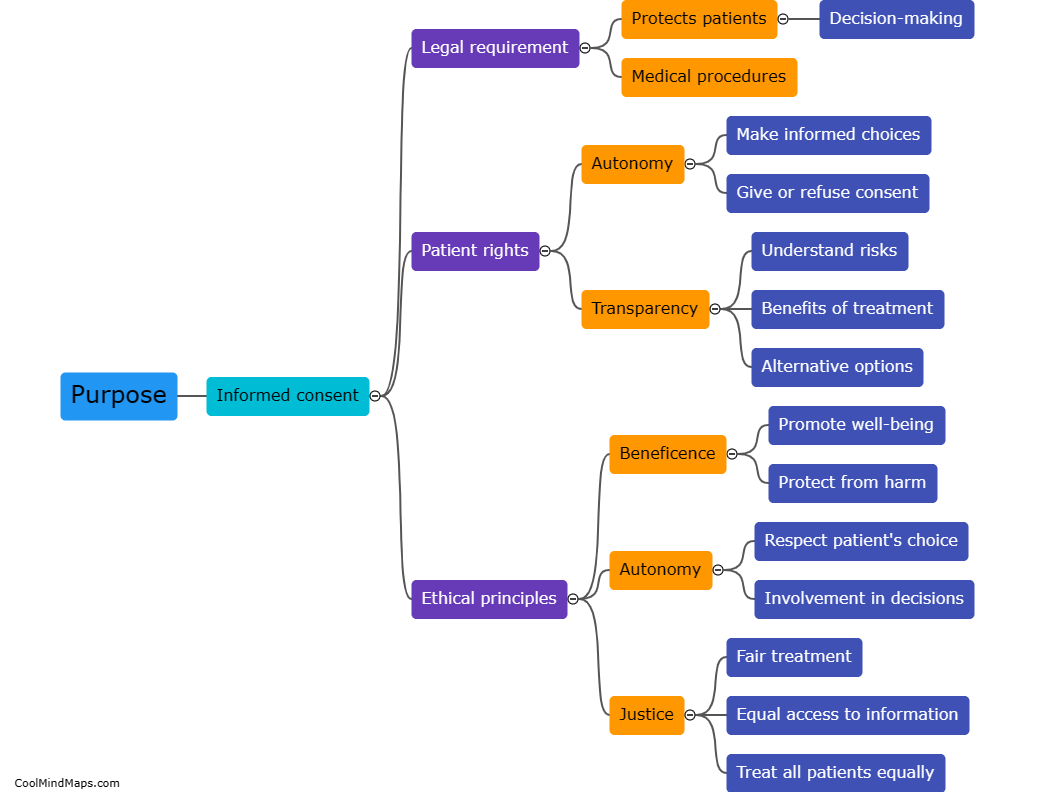
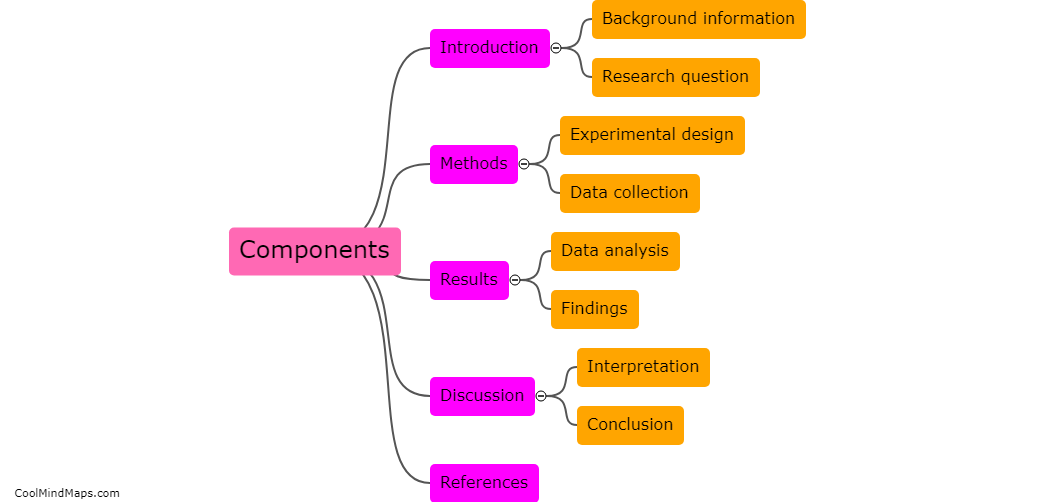
The purpose of a clinical study report (CSR) in clinical research is to provide a comprehensive and detailed account of a clinical trial's design, methodology, results, and conclusions. It serves as a crucial document that summarizes and communicates the key findings and outcomes of the study to regulatory authorities, ethics committees, and the scientific community. CSRs not only document the safety and efficacy of investigational products but also help investigators, researchers, and stakeholders make informed decisions for future clinical development and patient care. Furthermore, the CSR plays a vital role in ensuring transparency and accountability in clinical research, as it allows for examination, verification, and replication of the study by other researchers.
This mind map was published on 16 January 2024 and has been viewed 97 times.