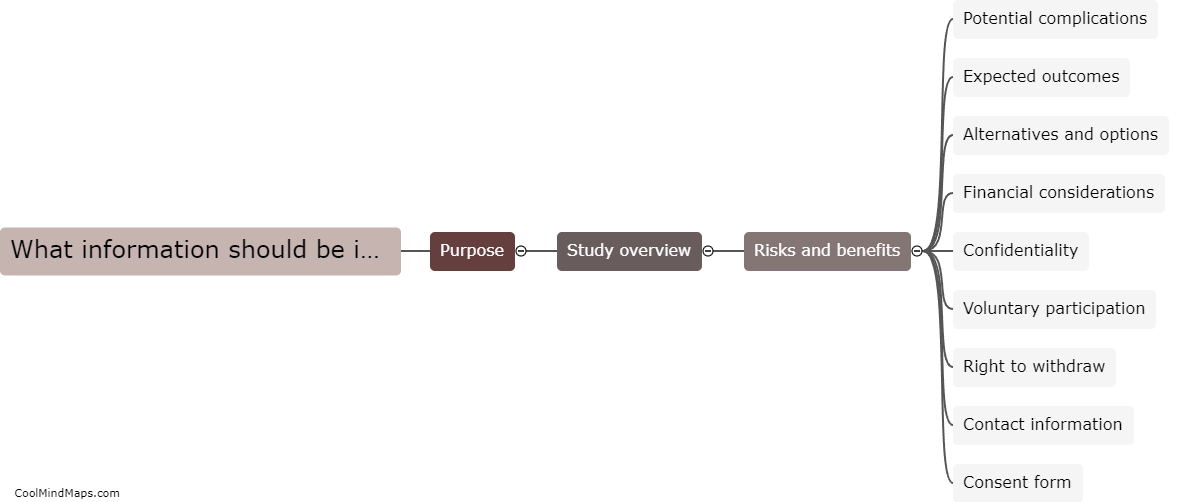
What information should be included in informed consent?
Informed consent is a crucial ethical principle in any setting that involves human subjects, such as medical research, clinical trials, or even routine medical procedures. It is important for individuals to have a complete understanding of the risks, benefits, and alternatives associated with the procedure or study they are about to engage in. Therefore, the information included in informed consent typically contains details about the purpose and nature of the procedure, the potential risks and benefits, the alternative options available, any financial costs or compensation involved, the extent of confidentiality and data protection measures, the voluntary nature of participation, the right to withdraw at any time, and contact information for further inquiries or concerns. Additionally, it should be presented in a clear and understandable manner, ensuring that the individual is fully aware and voluntarily agrees to participate.
This mind map was published on 5 December 2023 and has been viewed 89 times.