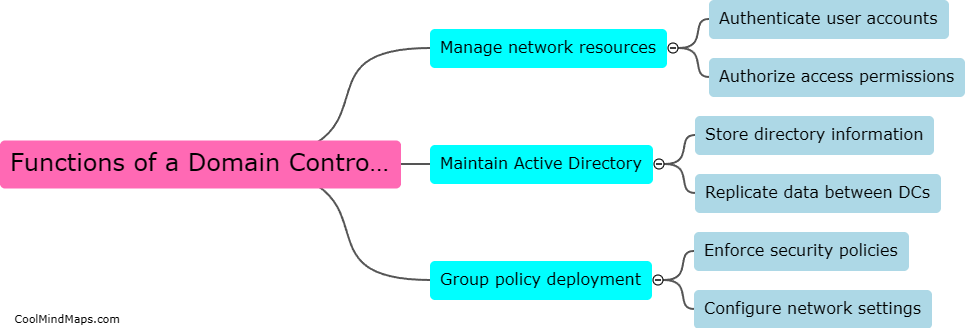
What are the types of CRF in the process?
There are two main types of CRF (Case Report Form) in the process: paper-based CRFs and electronic CRFs (eCRFs). Paper-based CRFs are physical forms that participants or healthcare professionals fill out manually. These forms are then collected, reviewed, and entered into a database by designated personnel. On the other hand, eCRFs are electronic versions of the CRFs that are filled out using computers or other digital devices. These forms can be accessed and completed online, allowing for real-time data collection and immediate data entry into the database. Both types of CRFs serve the purpose of capturing and collecting essential data during clinical trials, but eCRFs provide the advantages of efficiency, accuracy, and convenience in data management.

This mind map was published on 5 December 2023 and has been viewed 73 times.