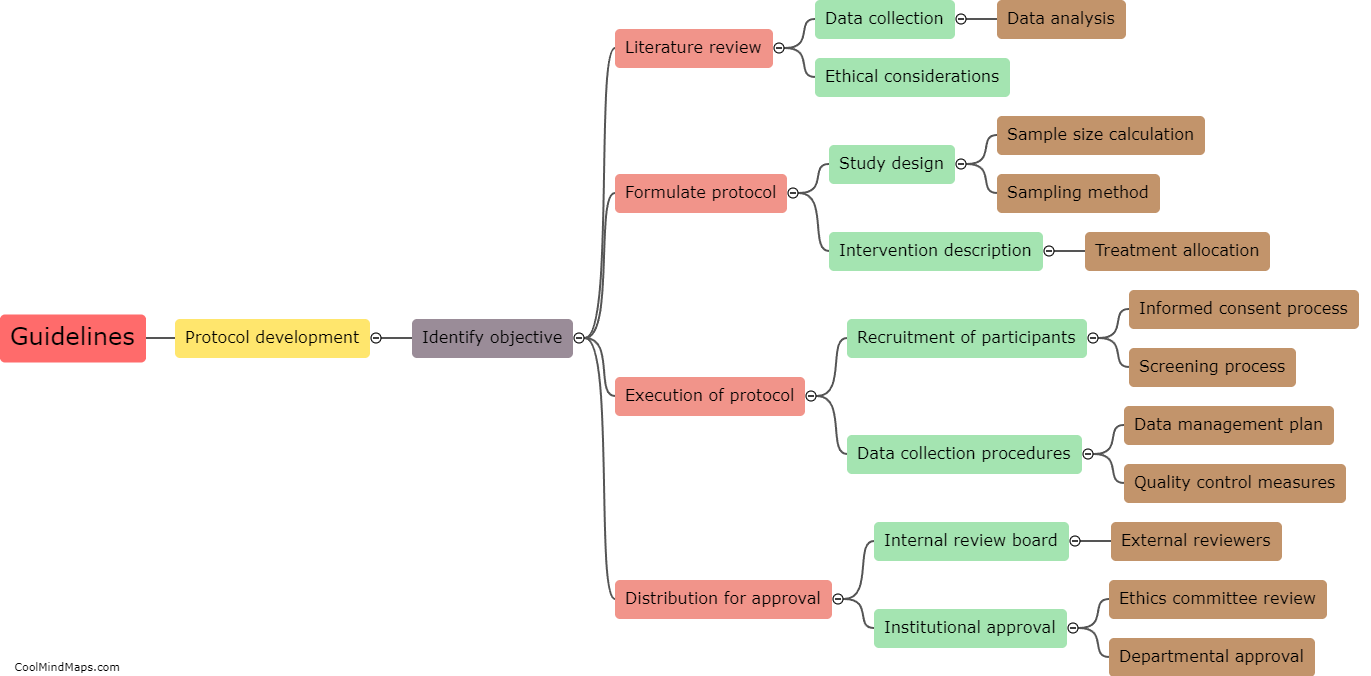
What are the guidelines for protocol development and approval?
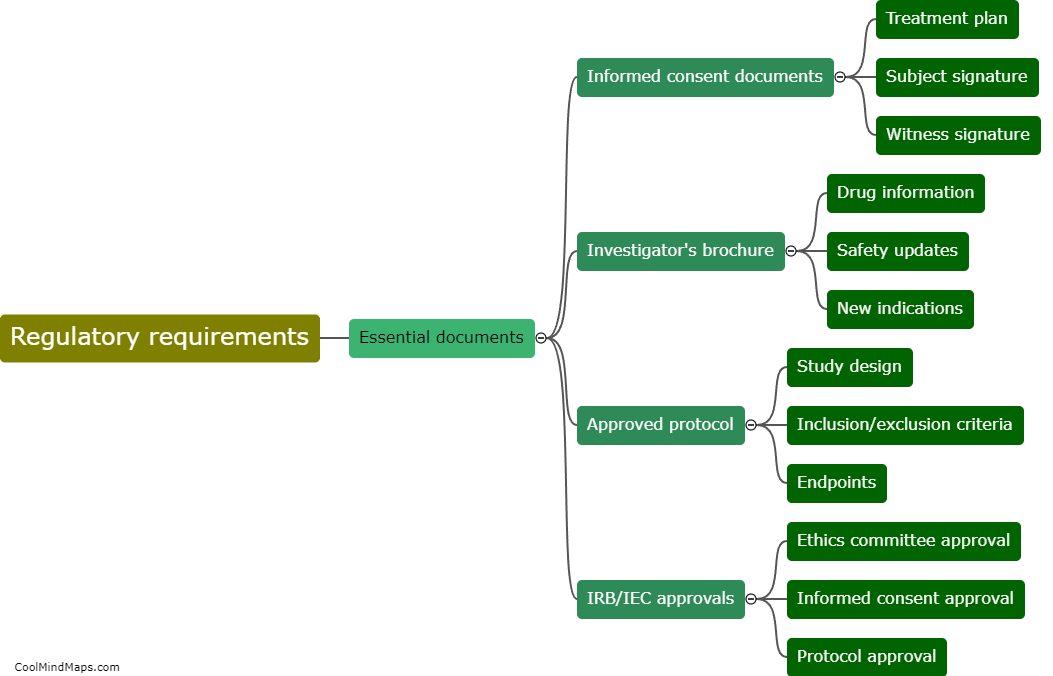
The guidelines for protocol development and approval serve as a framework to ensure that protocols are well-designed, rigorous, ethical, and align with the objectives of the study. These guidelines typically include various elements such as precise study objectives, inclusion and exclusion criteria, study methodology, data collection and analysis procedures, participant recruitment strategies, ethical considerations, and potential risks and benefits. The development process involves thorough planning, literature review, consultation with experts, and consideration of applicable regulations. Once the protocol is drafted, it generally undergoes a rigorous review and approval process by research ethics committees or institutional review boards to ensure participant safety, ethical conduct, and scientific validity. The approval ensures that the research complies with ethical and legal requirements and that the study design is well-reasoned and scientifically sound.
This mind map was published on 5 December 2023 and has been viewed 84 times.