What is the purpose of the informed consent process?
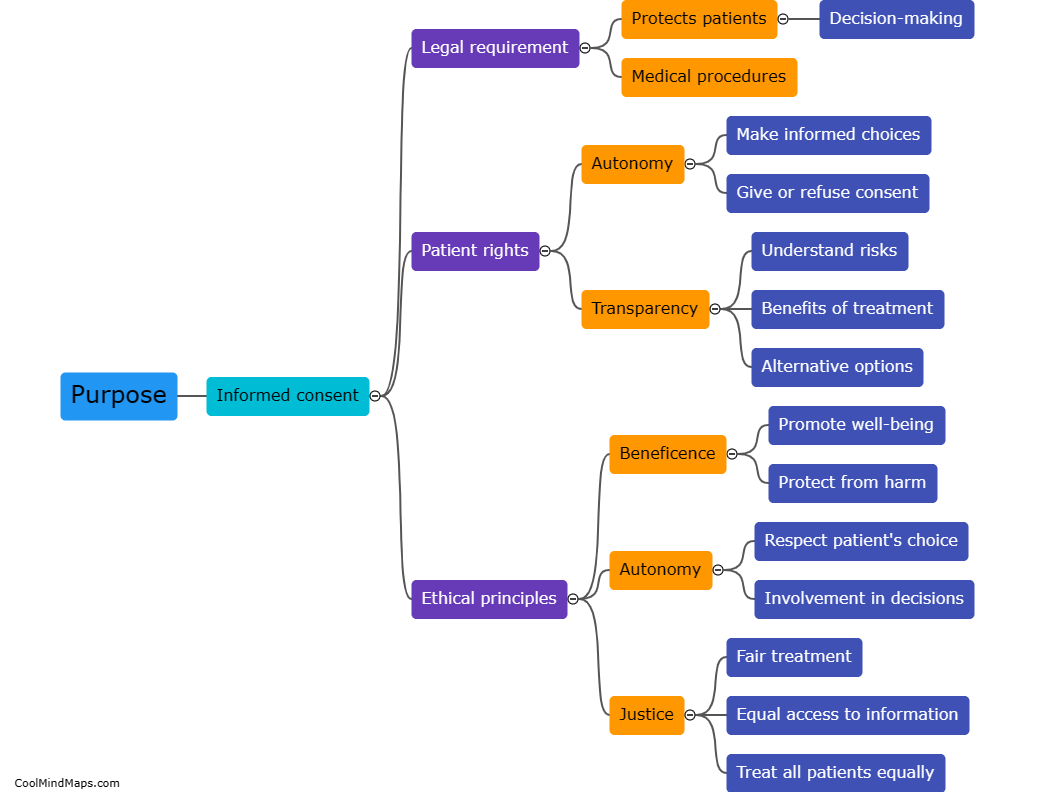
The purpose of the informed consent process is to ensure that individuals have sufficient understanding and knowledge about a particular medical procedure, treatment, or research study in order to make an informed decision. It involves providing individuals with relevant information regarding the risks, benefits, alternatives, and potential outcomes related to the procedure or study. By obtaining informed consent, healthcare practitioners and researchers respect the autonomy and rights of patients or subjects, allowing them to actively participate in decision-making and ensuring that their consent is voluntary, uncoerced, and based on accurate information. Additionally, the informed consent process helps foster trust and good communication between healthcare providers, researchers, and individuals, promoting ethical and respectful practices in medical settings.
This mind map was published on 16 January 2024 and has been viewed 95 times.