How does Uranium decay over time?
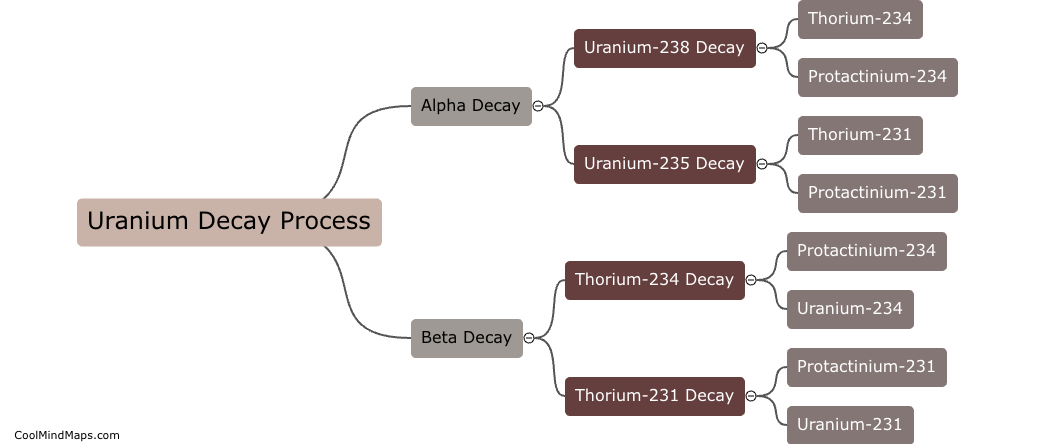
Uranium decays over time through a process known as radioactive decay. Specifically, uranium-238, the most common isotope of uranium, undergoes alpha decay, emitting an alpha particle (two protons and two neutrons) from its nucleus to transform into thorium-234. This is just the first step in a series of decays that eventually lead to the stable isotope lead-206. The entire decay process for uranium-238 takes billions of years to complete, with uranium-238 having a half-life of about 4.5 billion years.
This mind map was published on 22 March 2024 and has been viewed 85 times.