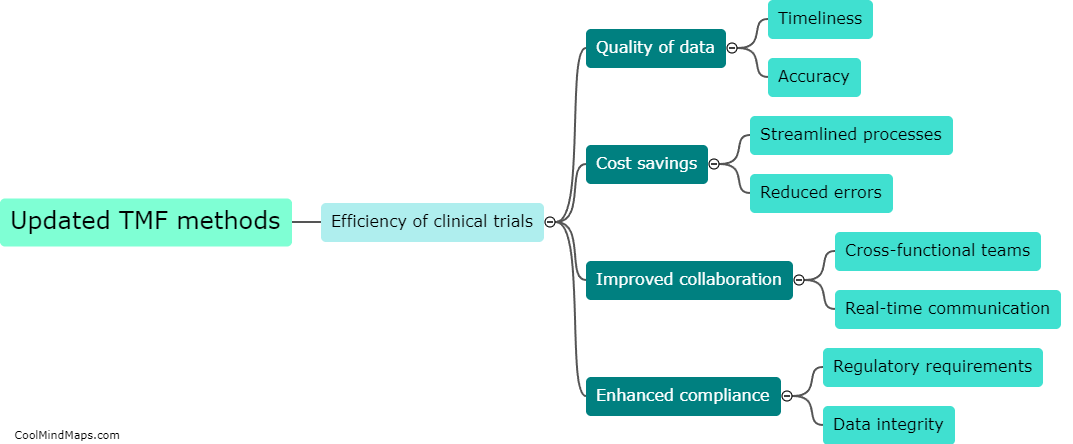
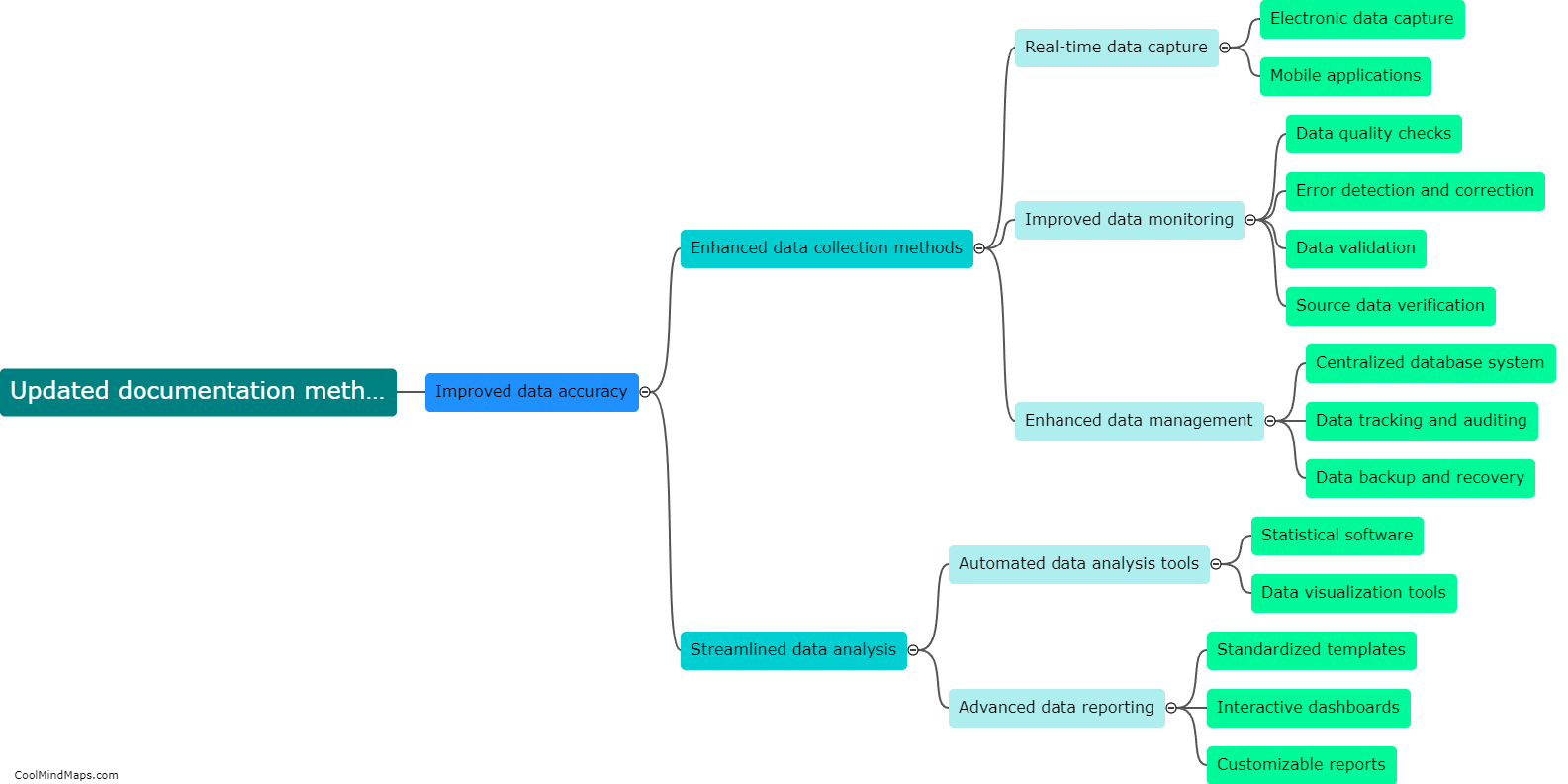
What is the efficiency of clinical trials with updated essential documentation methods?
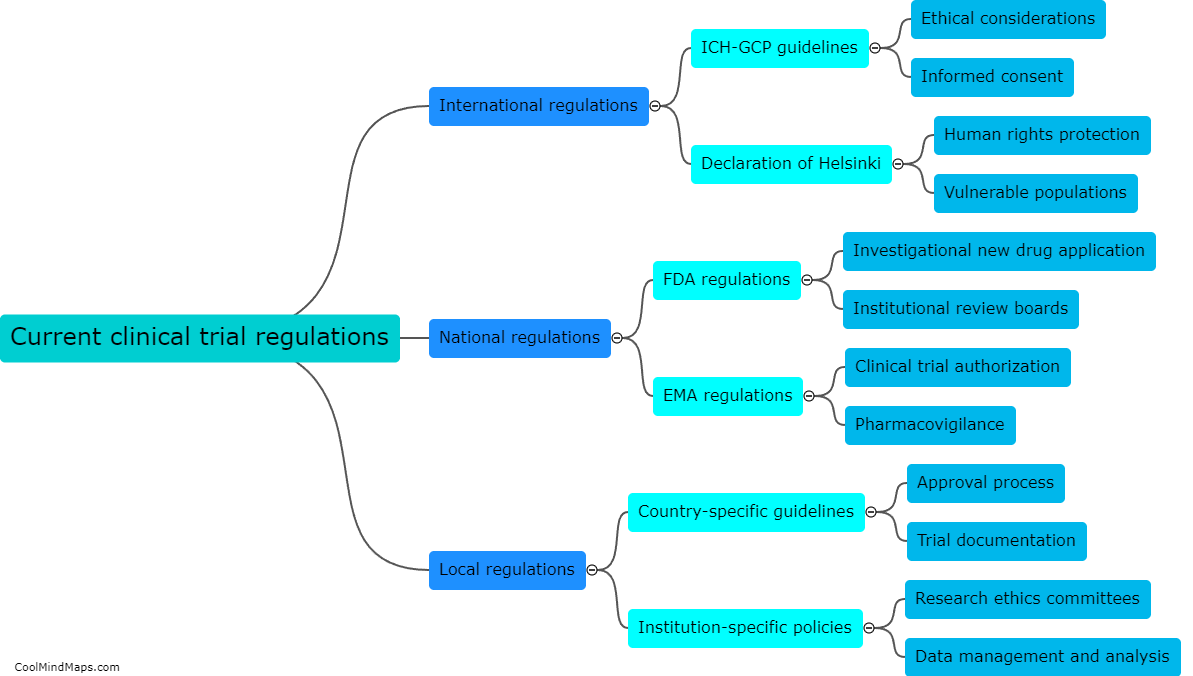
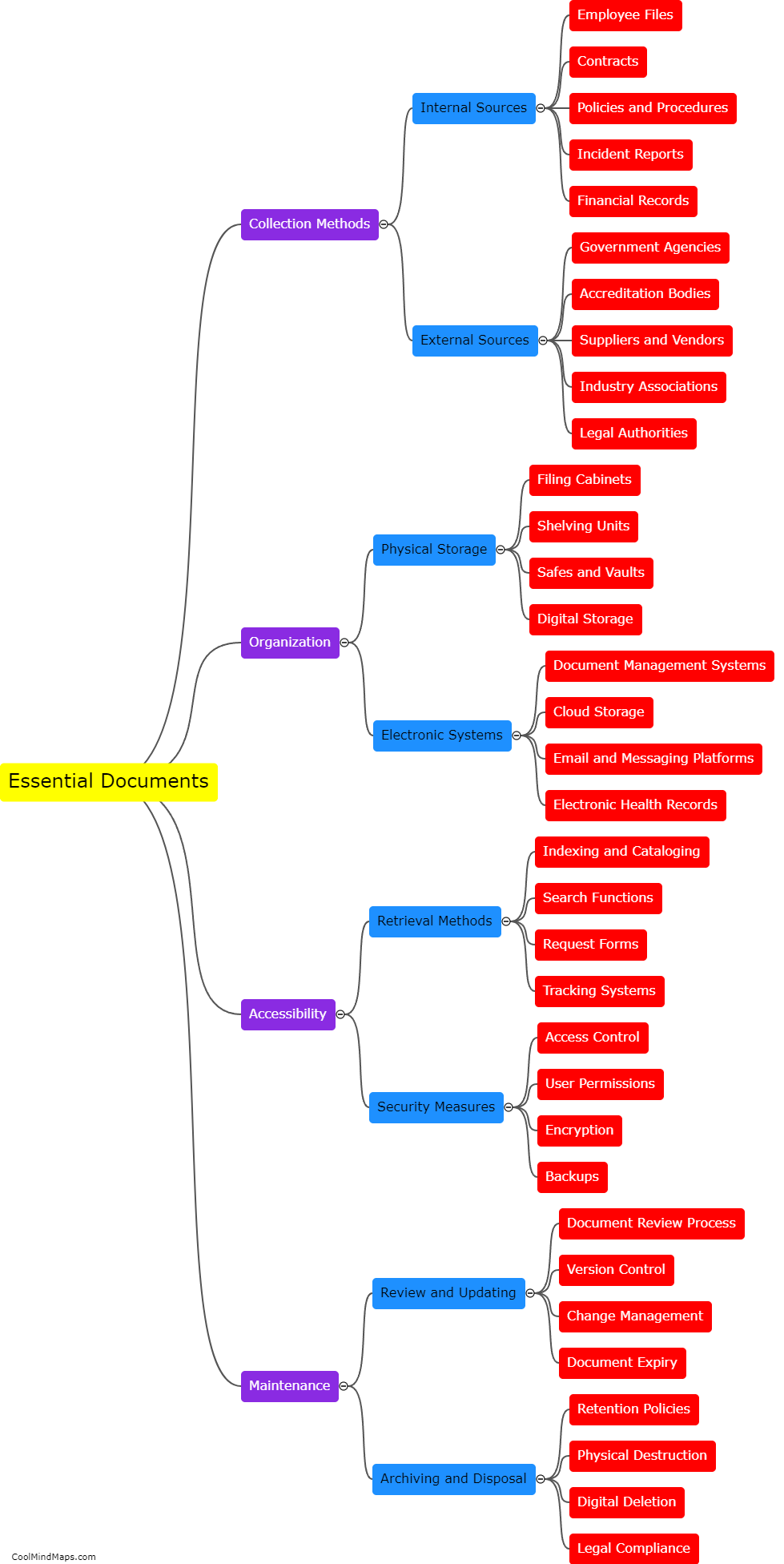
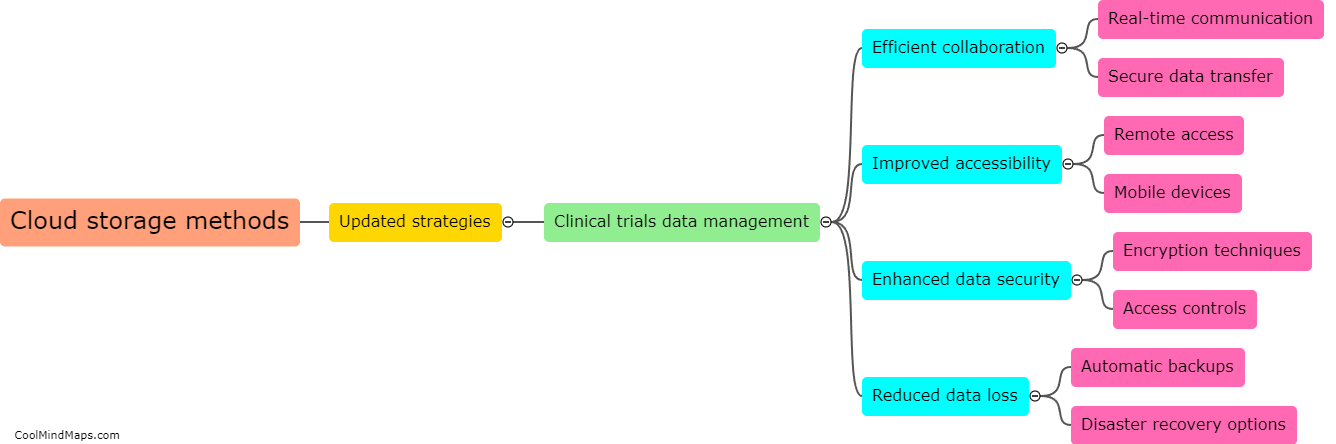
Clinical trials are vital for testing the safety and efficacy of new drugs and medical devices before they are approved for public use. Ensuring the efficiency of these trials is crucial to accelerate the development of innovative treatments. With the advent of updated essential documentation methods, such as electronic data capture (EDC) systems and electronic trial master files (eTMFs), the efficiency of clinical trials has significantly improved. These advanced technologies streamline the documentation process, facilitate real-time data collection, and enhance overall trial management. By eliminating the need for paper-based records, these methods reduce human error and simplify data analysis, leading to quicker and more accurate results. Consequently, the use of updated essential documentation methods in clinical trials has considerably enhanced efficiency and expedited the development of life-saving medications and treatments.

This mind map was published on 7 January 2024 and has been viewed 91 times.