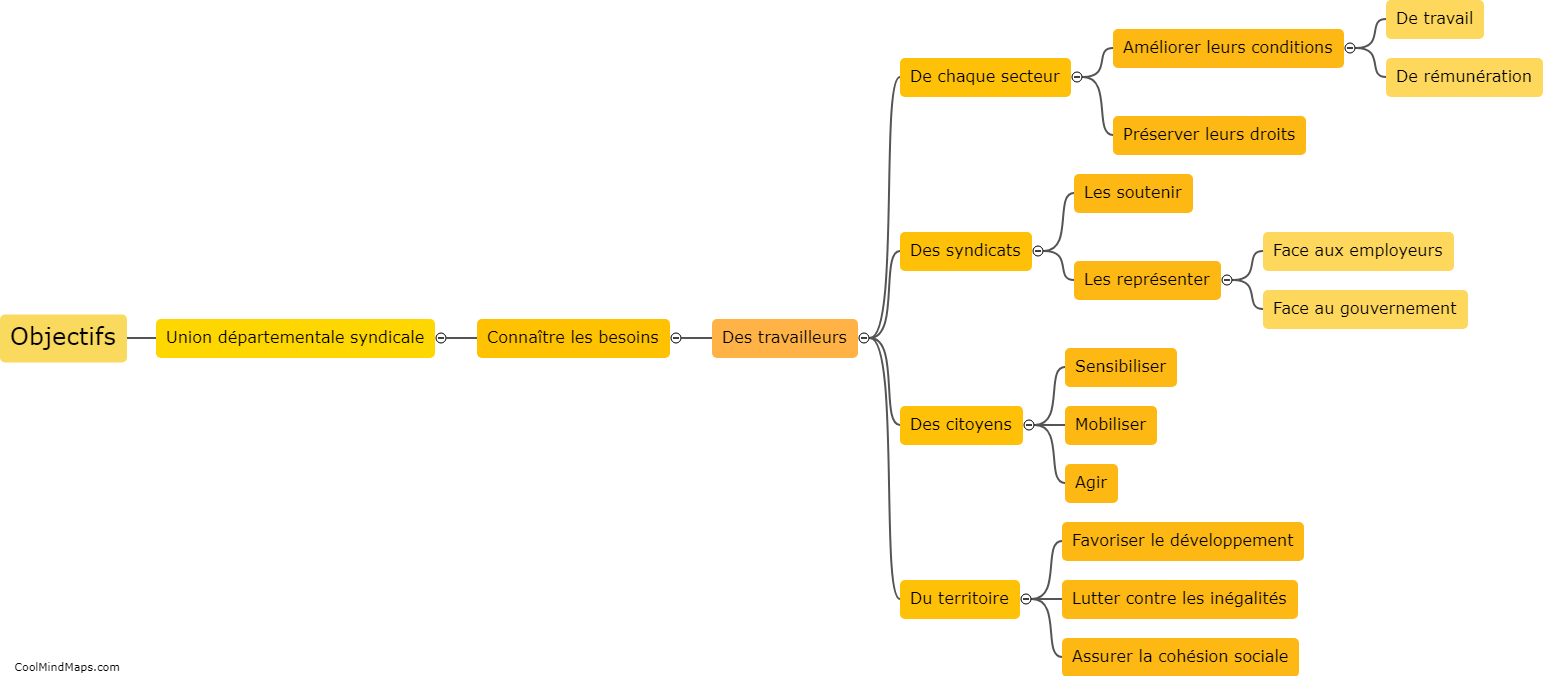
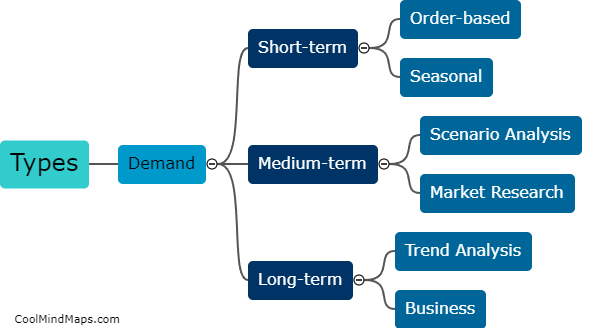
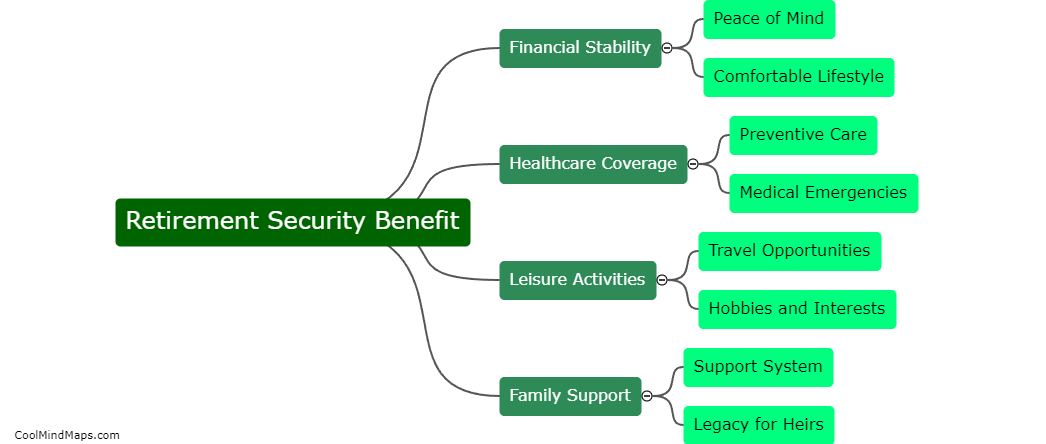
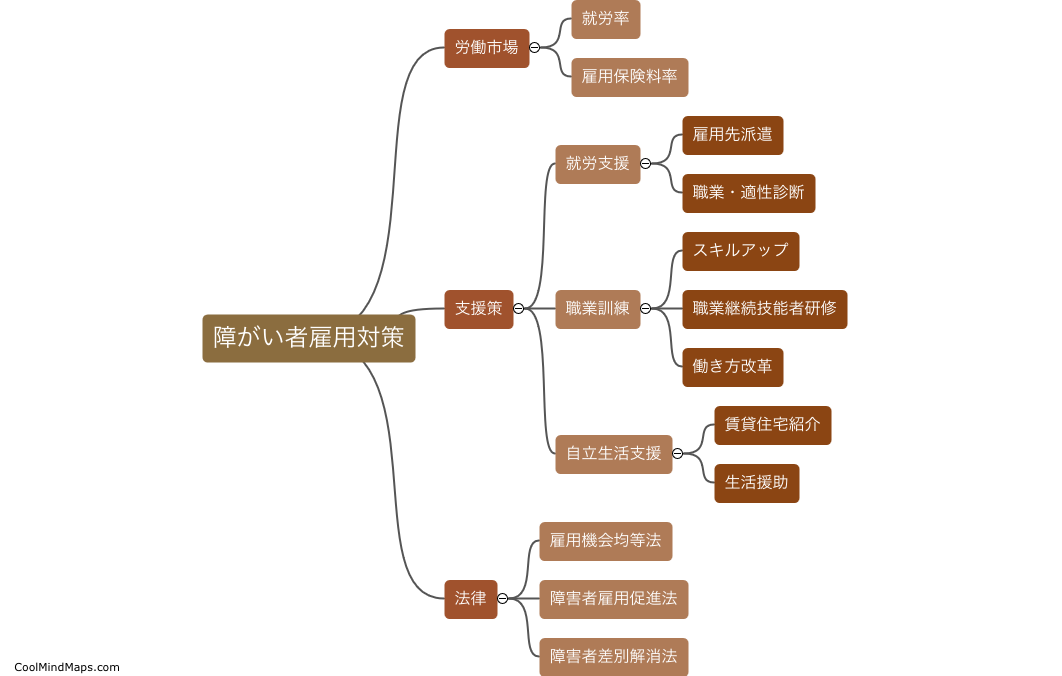
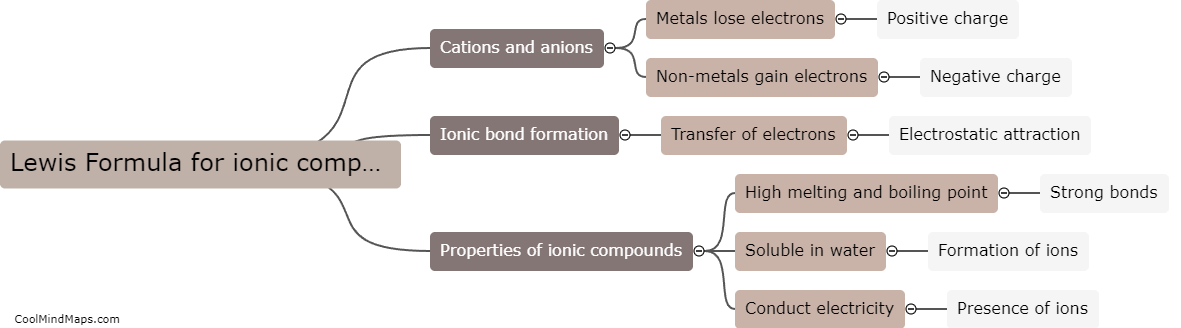
Lewis Formula for ionic compounds
The Lewis formula, also known as the electron-dot formula, is a diagrammatic representation of the valence electrons in an atom or molecule. In the case of ionic compounds, the Lewis formula shows the transfer of electrons from one atom to another and how the resulting ions are held together by electrostatic forces. For example, the Lewis formula for sodium chloride (NaCl) shows that sodium donates its single valence electron to chlorine, which gains a full outer shell and becomes a negatively charged ion (Cl-), while sodium becomes positively charged (Na+). The resulting ions attract each other to form a crystal lattice structure. The Lewis formula is a useful tool for understanding the properties of ionic compounds, as well as for predicting the charges and properties of ions in solution.
This mind map was published on 11 June 2023 and has been viewed 109 times.