What is Kohlrausch law of independent migration of ions?
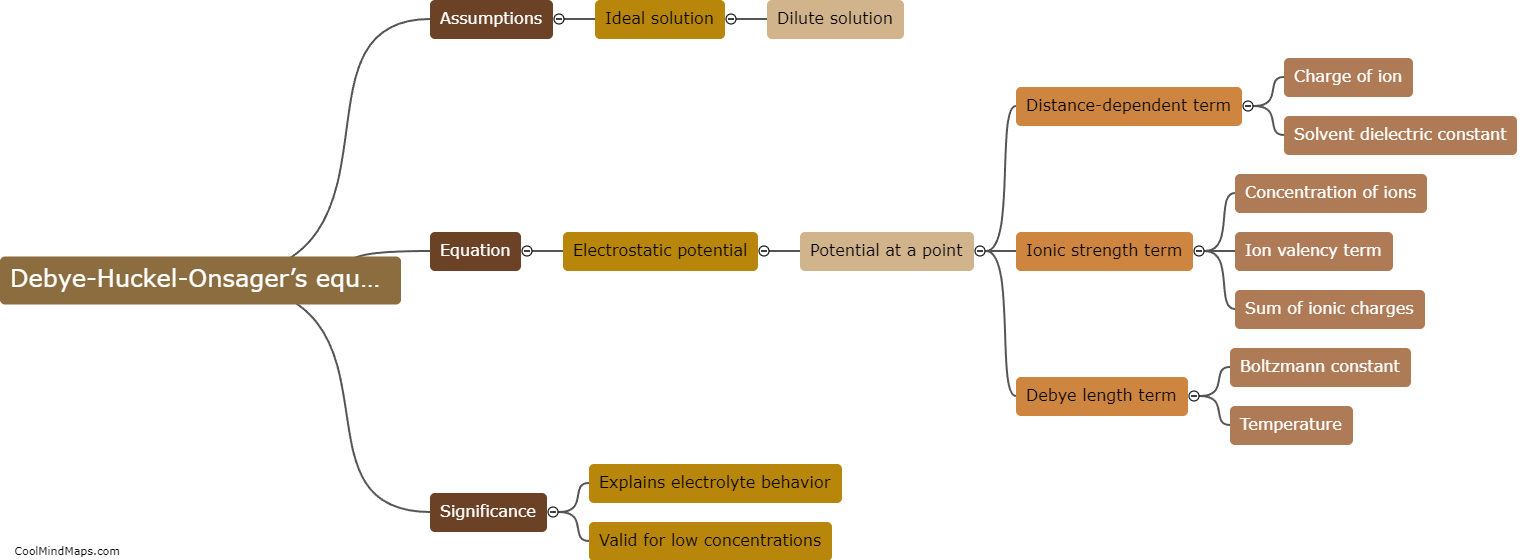
The Kohlrausch law of independent migration of ions, named after German physicist Friedrich Kohlrausch, is a principle that relates to the measurement of electrical conductivity in electrolyte solutions. According to this law, the total equivalent conductance of an electrolyte at infinite dilution is the sum of the individual contributions from its constituent ions. This means that the ions present in the solution move independently of each other, with their own characteristic speeds of migration. Kohlrausch's law allows scientists to calculate the conductance of individual ions, providing valuable insights into the behavior and properties of electrolytes. This law has significant applications in various fields such as chemistry, electrochemistry, and materials science.

This mind map was published on 31 August 2023 and has been viewed 170 times.