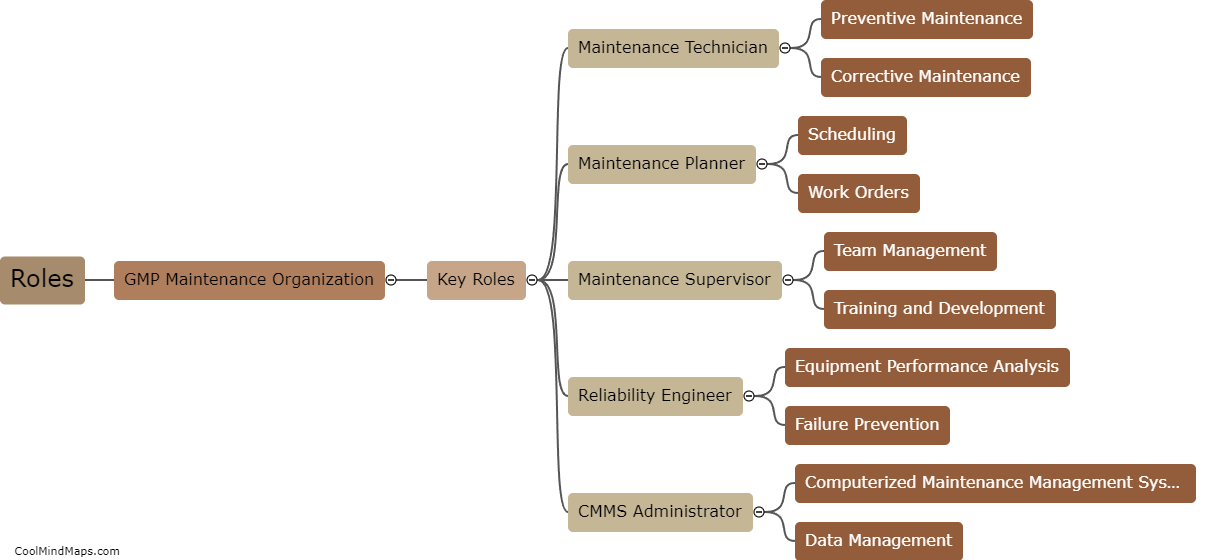
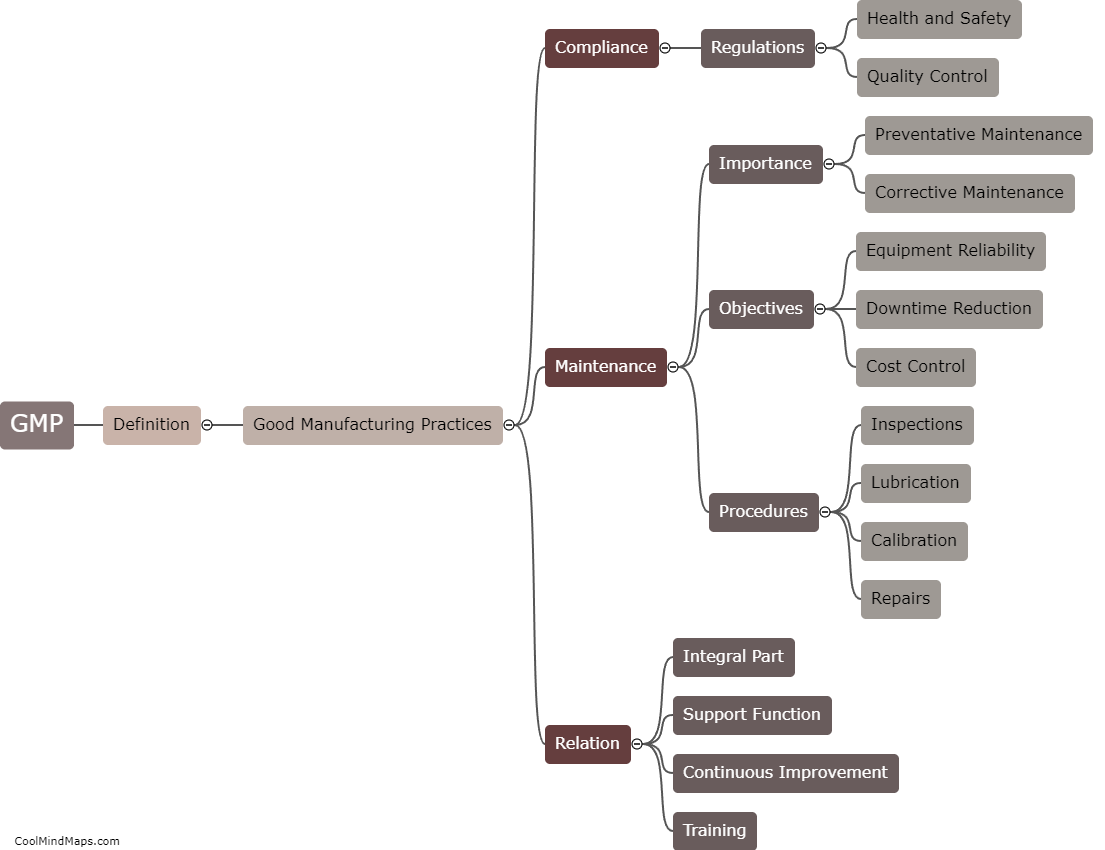
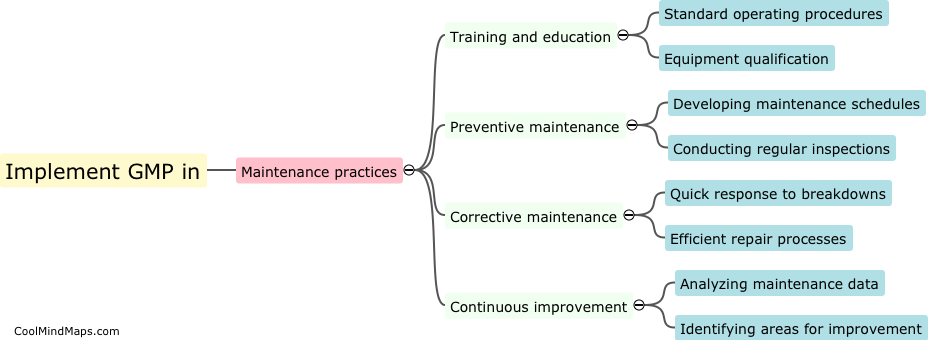
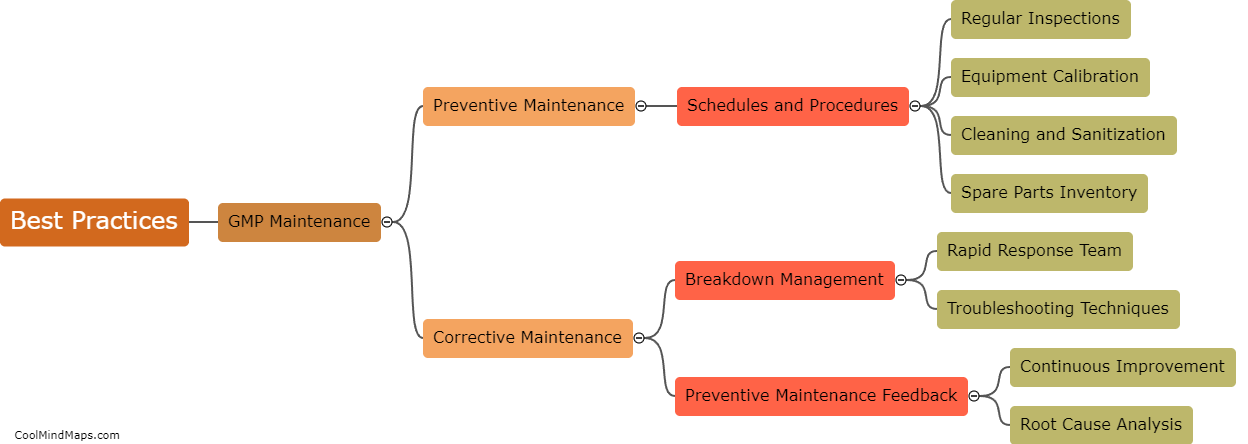
What are the best practices for GMP maintenance in pharmaceutical manufacturing?
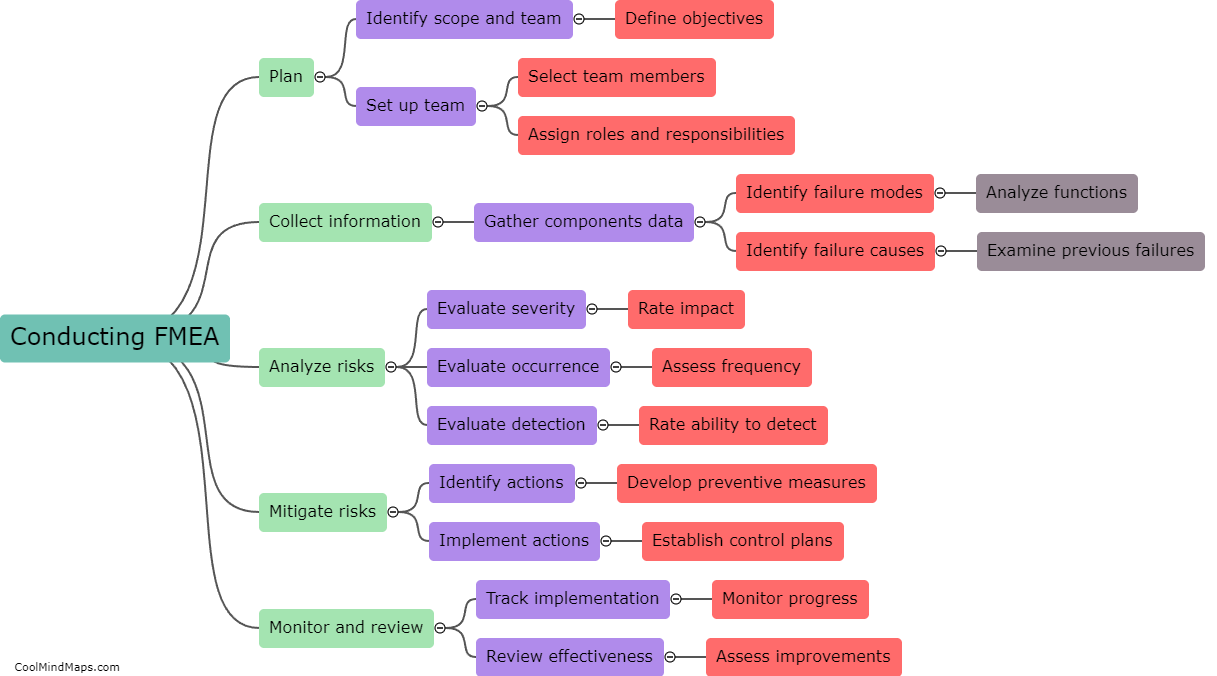
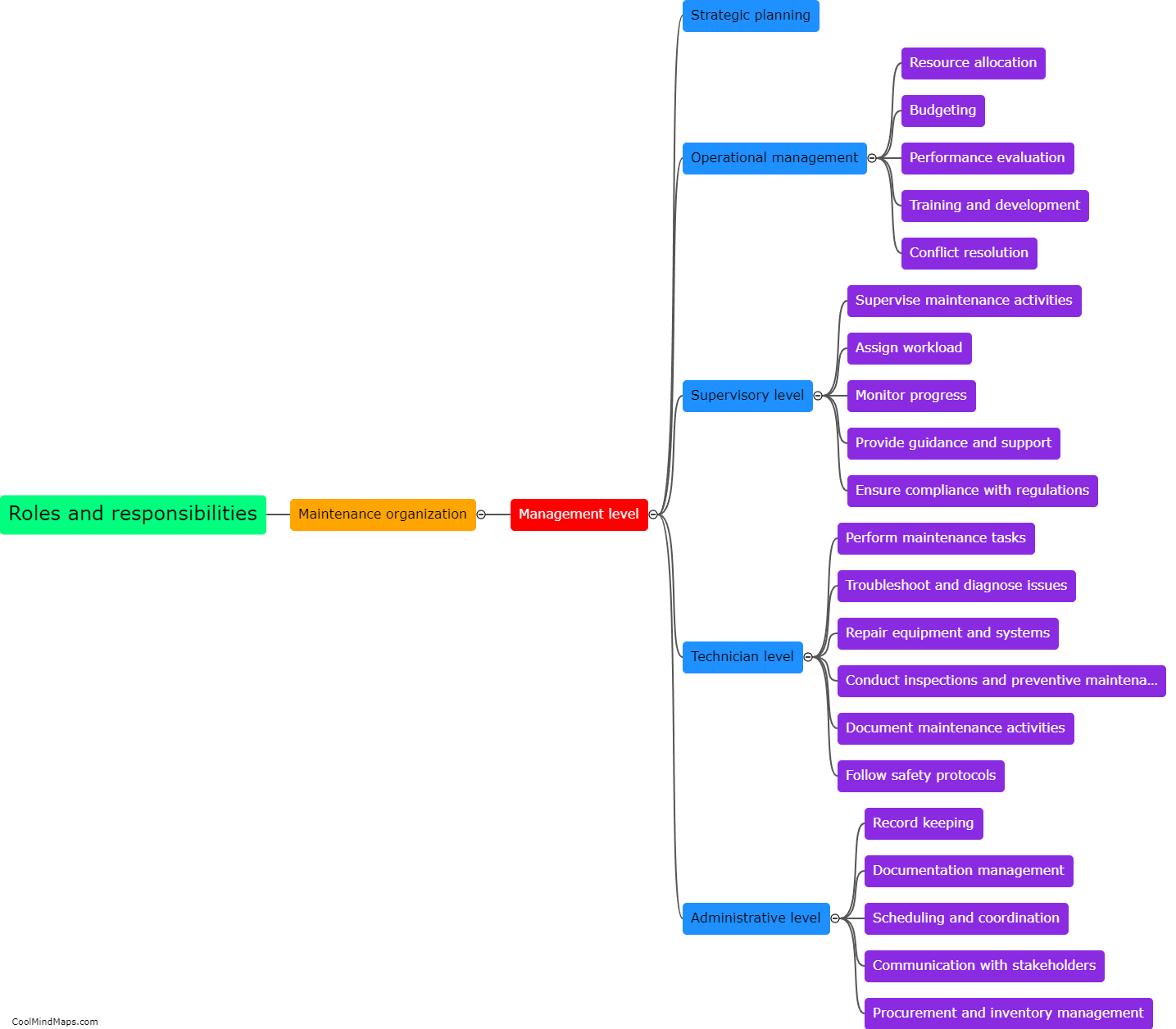
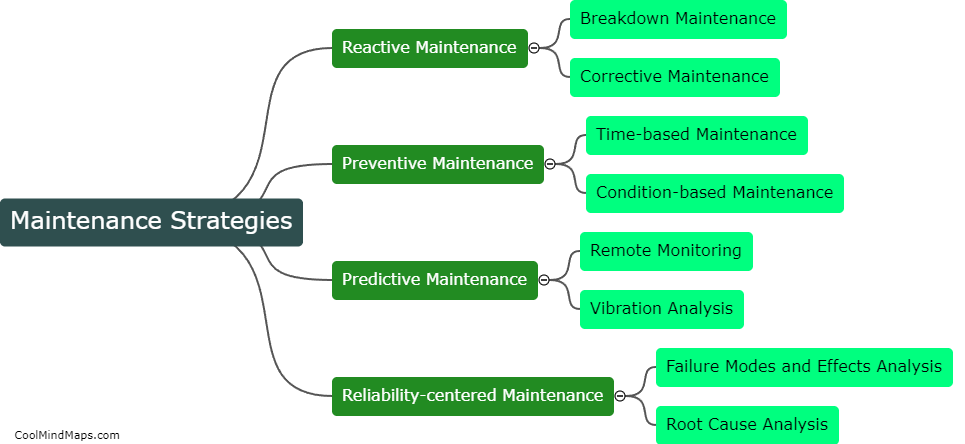
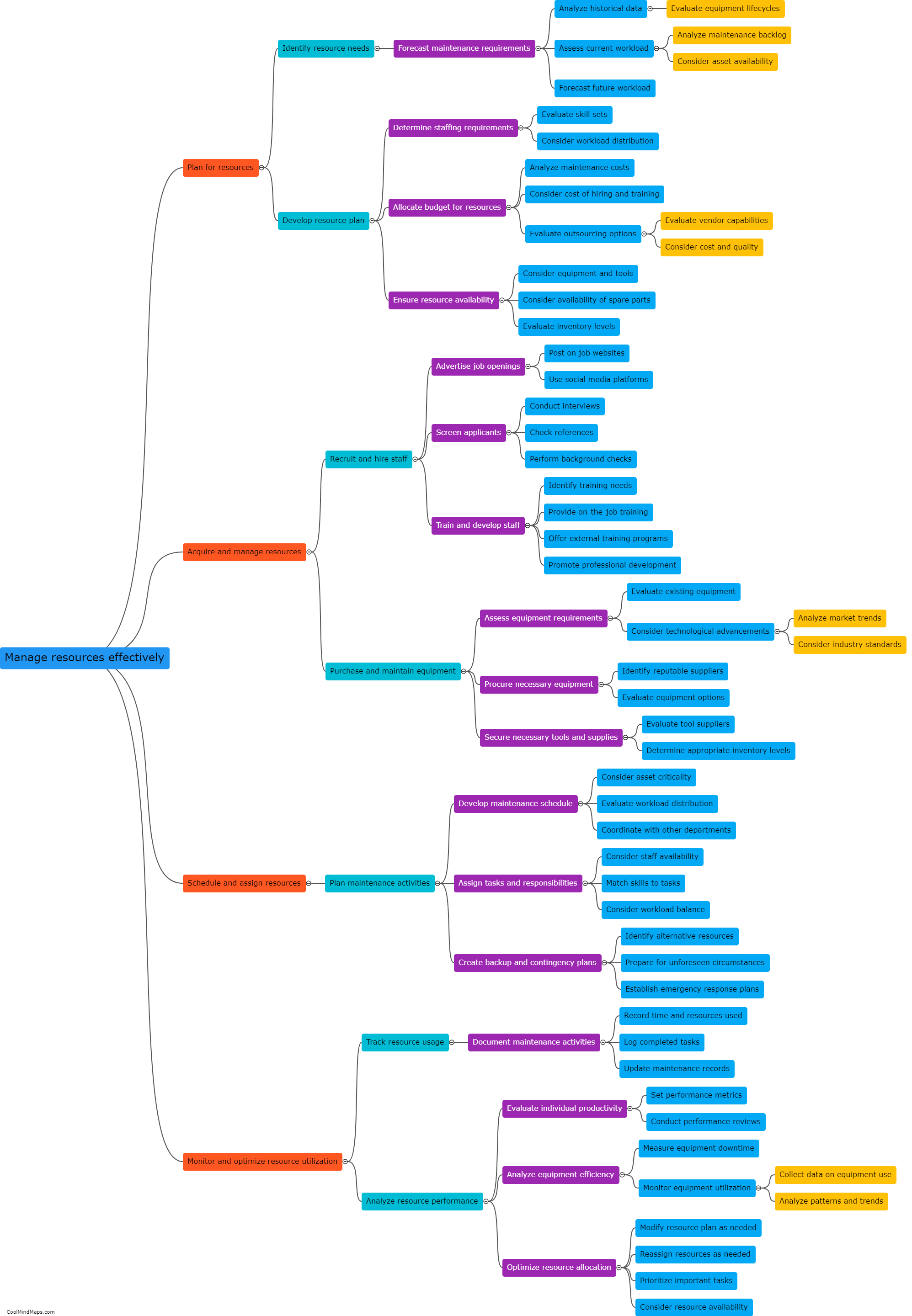
Maintaining good manufacturing practices (GMP) is crucial in the pharmaceutical manufacturing industry to ensure the safety, quality, and efficacy of drugs. Several best practices can be followed to ensure effective GMP maintenance in this sector. Firstly, regular and routine inspection and monitoring of equipment and facilities should be conducted to detect and address any potential issues promptly. Maintenance procedures and schedules must be clearly documented and followed to ensure equipment remains in optimal condition. Implementation of a robust preventive maintenance program, including calibration and validation procedures, is essential. Ongoing staff training and education on GMP practices should be provided to ensure compliance. Additionally, a robust documentation system to record all maintenance activities and deviations is crucial for traceability and regulatory compliance. By adhering to these best practices, pharmaceutical manufacturers can maintain the highest standards of quality, minimize risks, and meet regulatory requirements.
This mind map was published on 26 November 2023 and has been viewed 110 times.