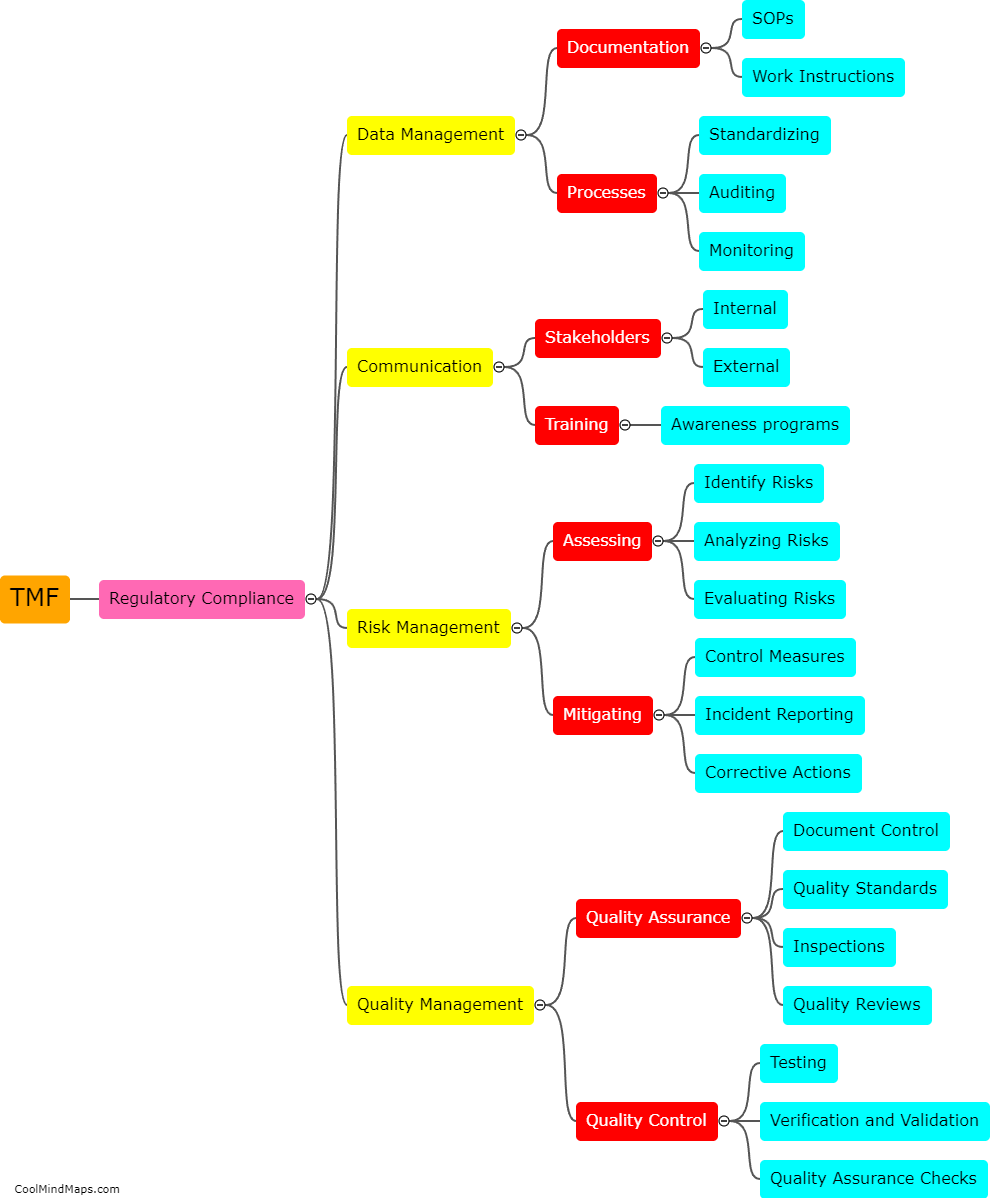
How does TMF contribute to ensuring regulatory compliance?
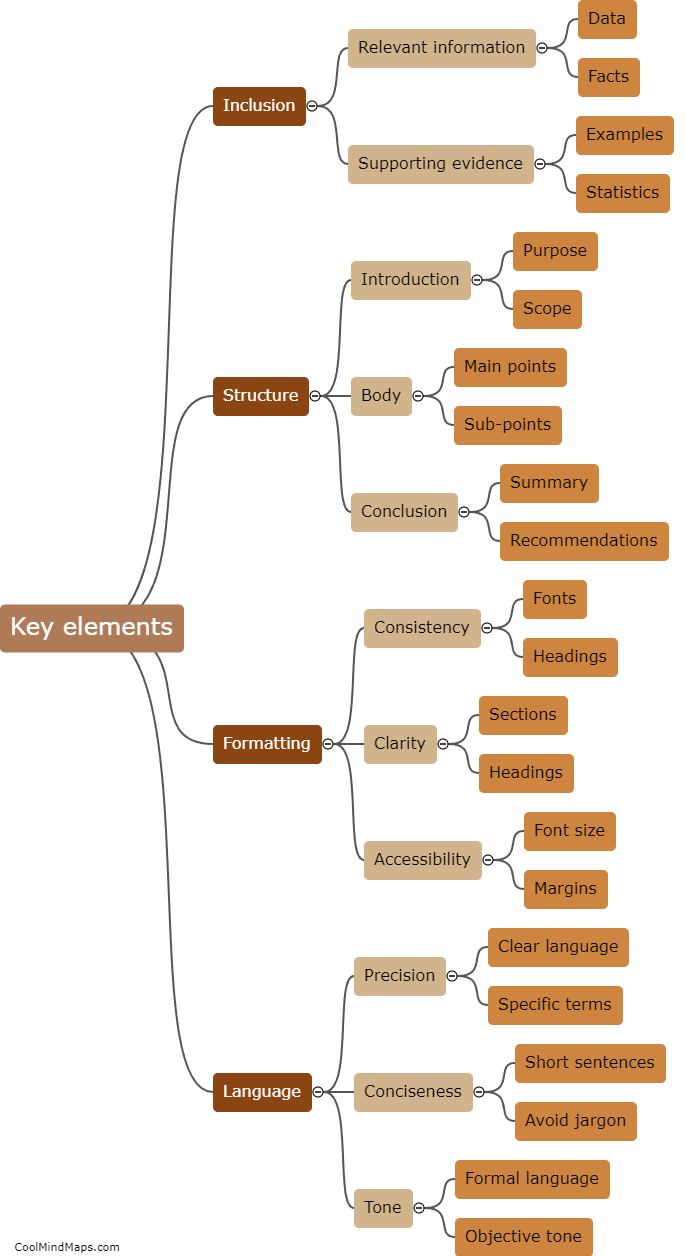
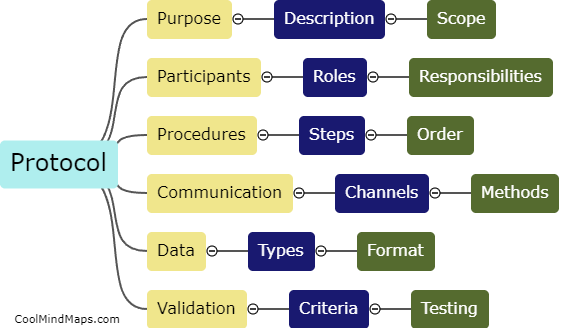
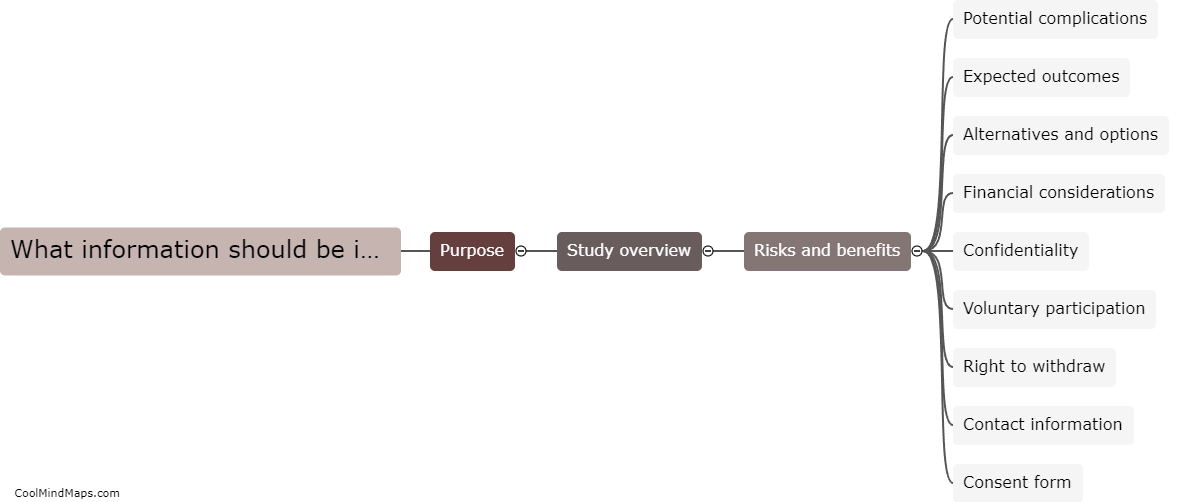
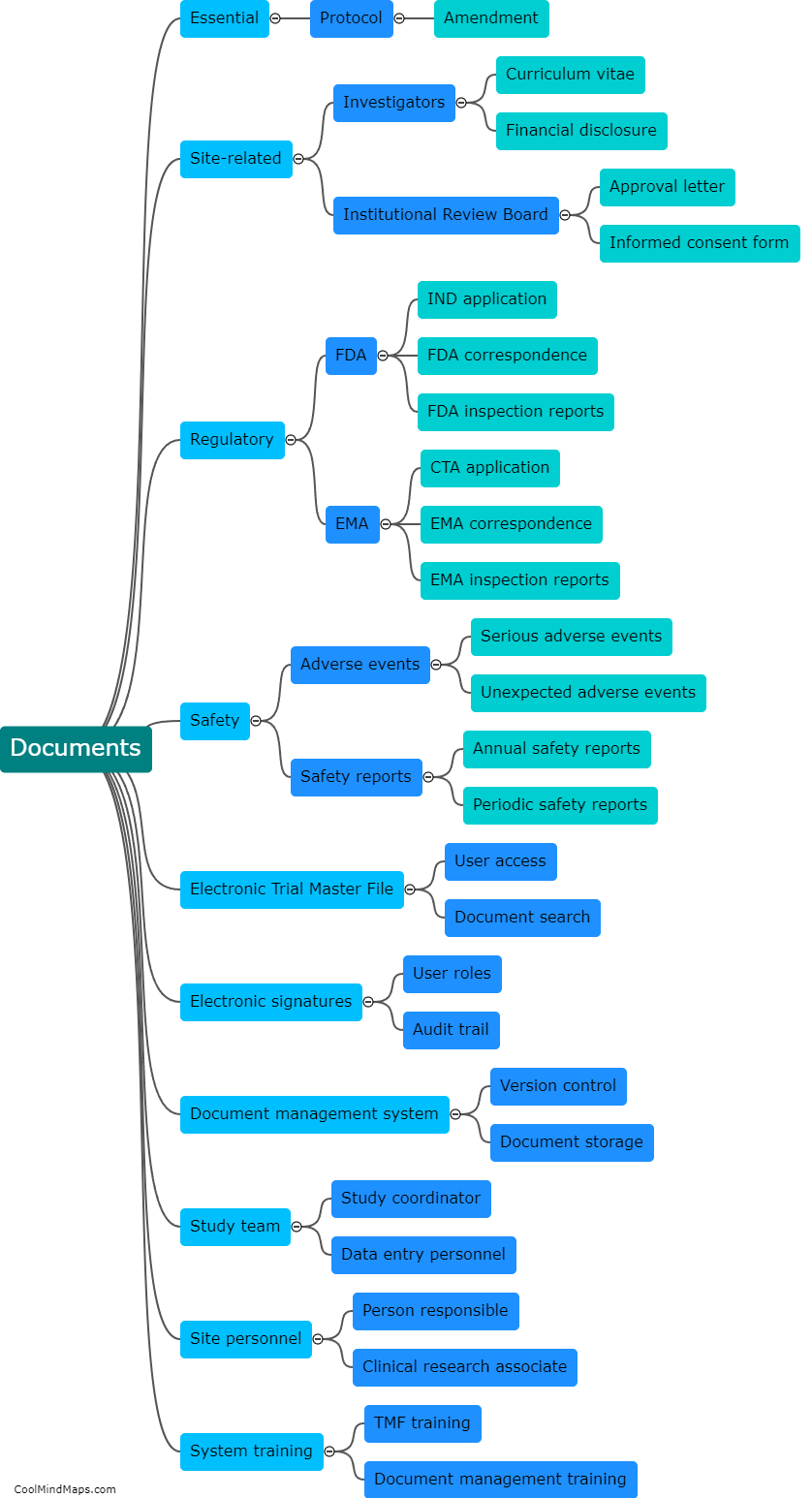
TMF (Trial Master File) plays a crucial role in ensuring regulatory compliance within clinical trials and research studies. The TMF serves as a centralized repository for all the essential documentation related to the trial, including regulatory documents, study protocols, informed consent forms, and study reports, among others. By maintaining an organized and complete TMF, regulatory authorities can easily review and audit the documentation, ensuring that all necessary regulatory requirements are met. A well-maintained TMF helps demonstrate that the trial is conducted in accordance with applicable regulations, enhances transparency, and enables efficient monitoring, thereby contributing significantly to ensuring regulatory compliance.
This mind map was published on 5 December 2023 and has been viewed 93 times.