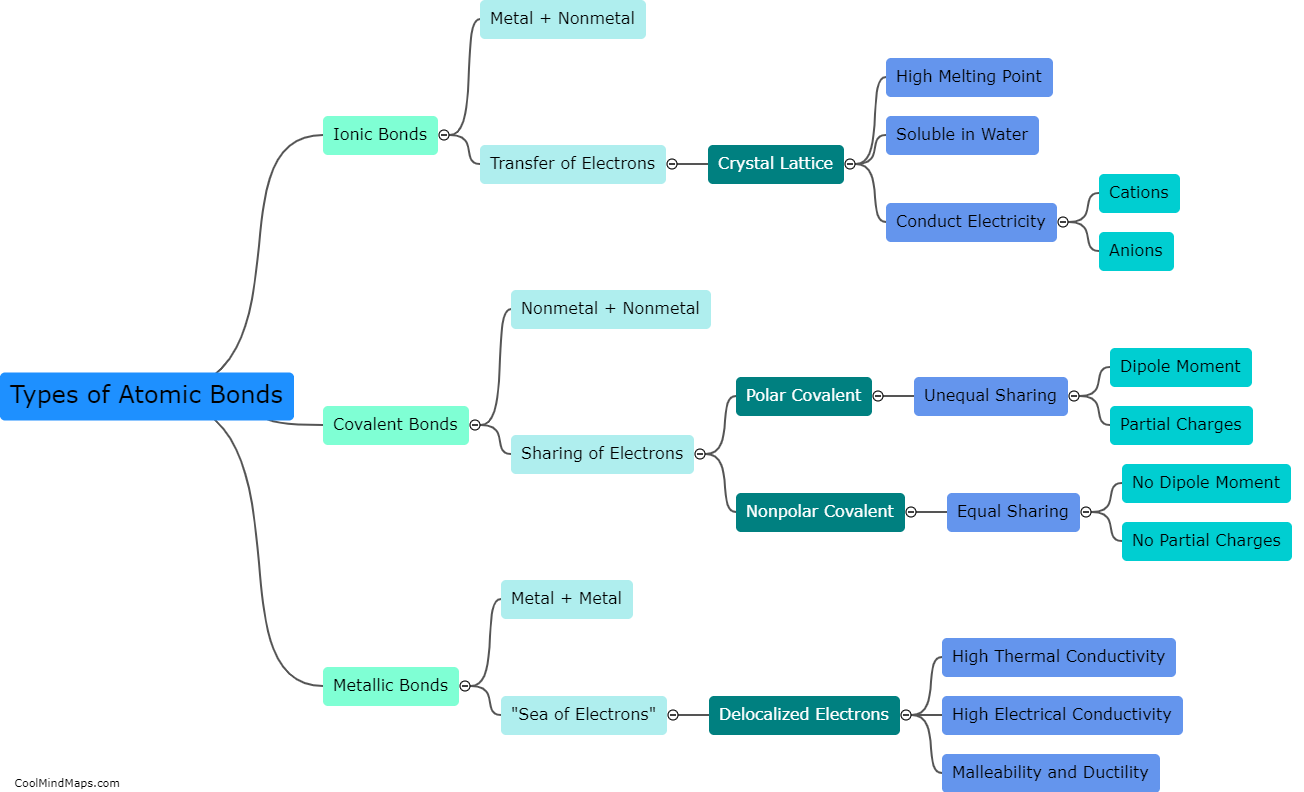
What are the types of atomic bonds?
There are three main types of atomic bonds that occur between atoms: ionic, covalent, and metallic bonds. Ionic bonds result from the transfer of electrons between atoms, creating ions with opposite charges that are attracted to each other. Covalent bonds involve atoms sharing electrons to achieve a more stable electron configuration. This type of bond occurs when atoms of nonmetals or similar electronegativities are involved. Metallic bonds occur in metals and are characterized by the sharing of electrons among a sea of delocalized electrons, creating a strong bond that allows for the high conductivity and malleability of metals. Each type of atomic bond has distinct properties and plays a crucial role in the formation and stability of various chemical compounds and structures.
This mind map was published on 25 October 2023 and has been viewed 163 times.