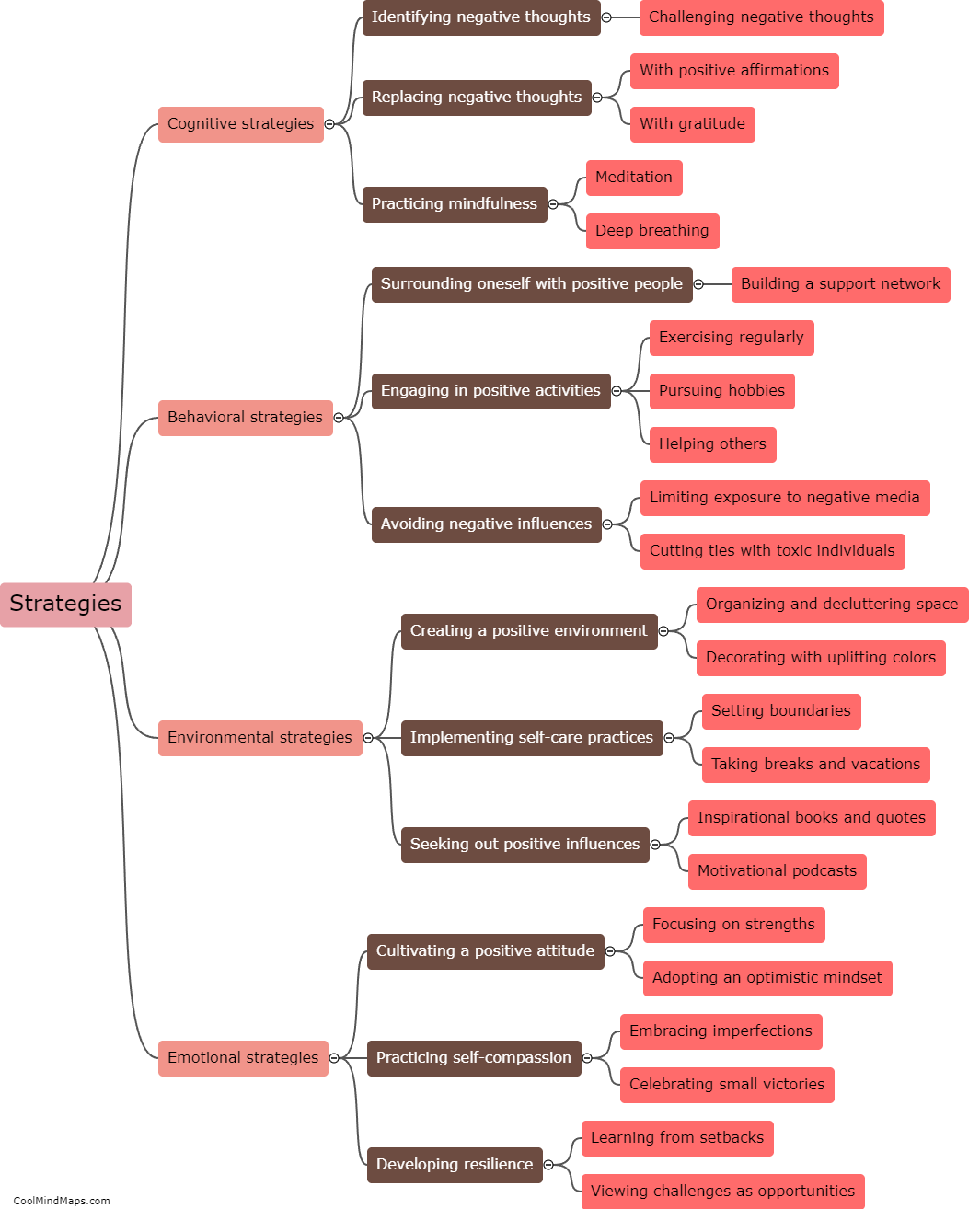
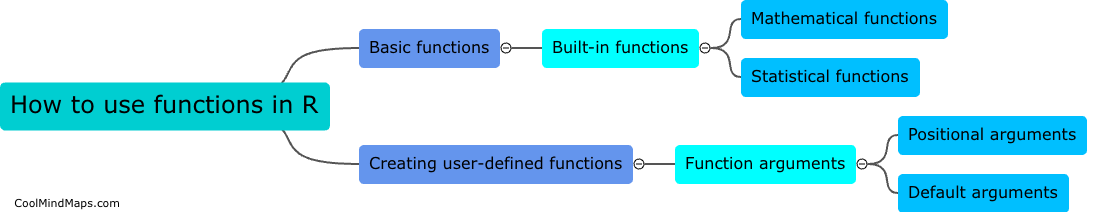
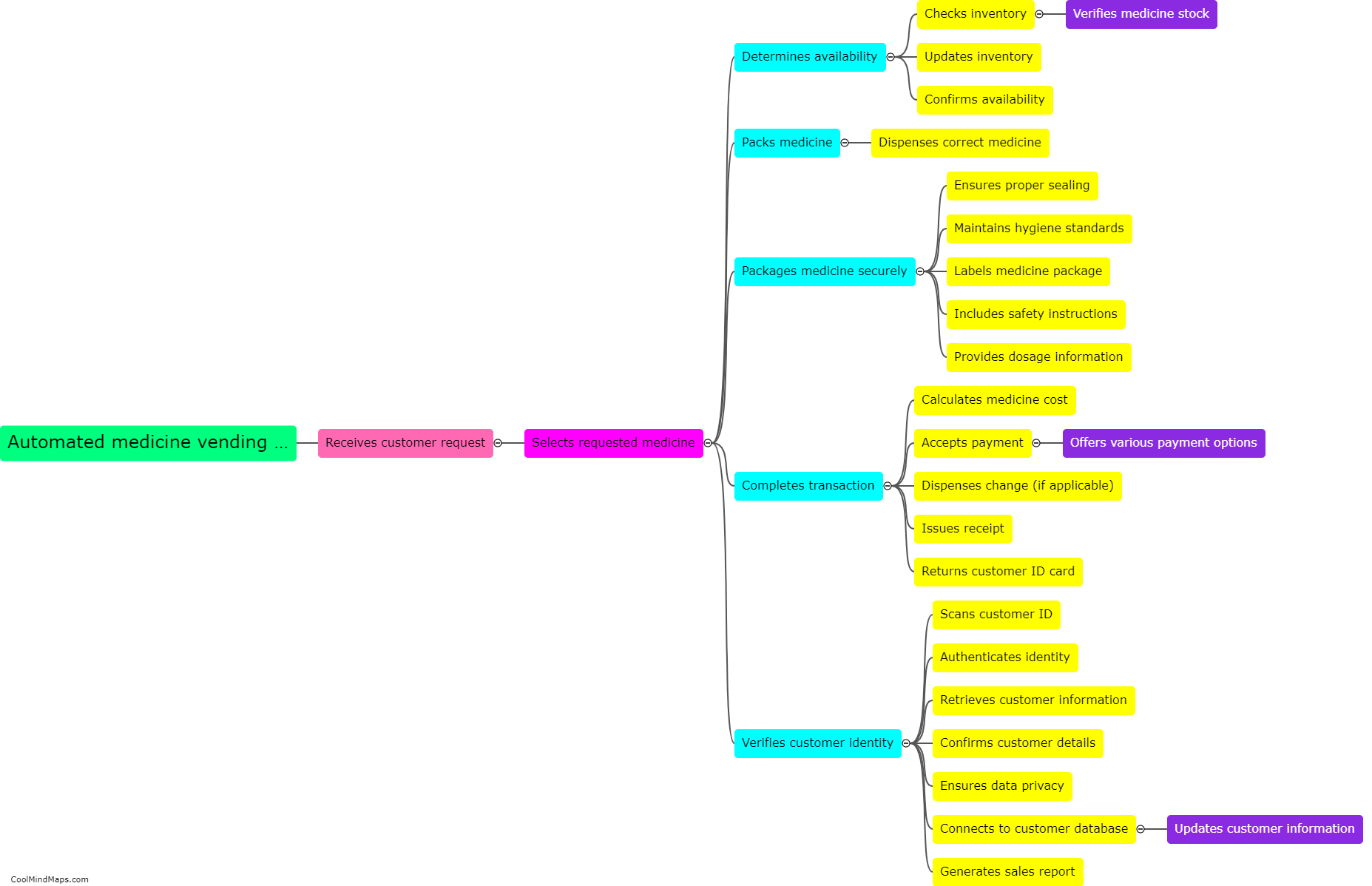
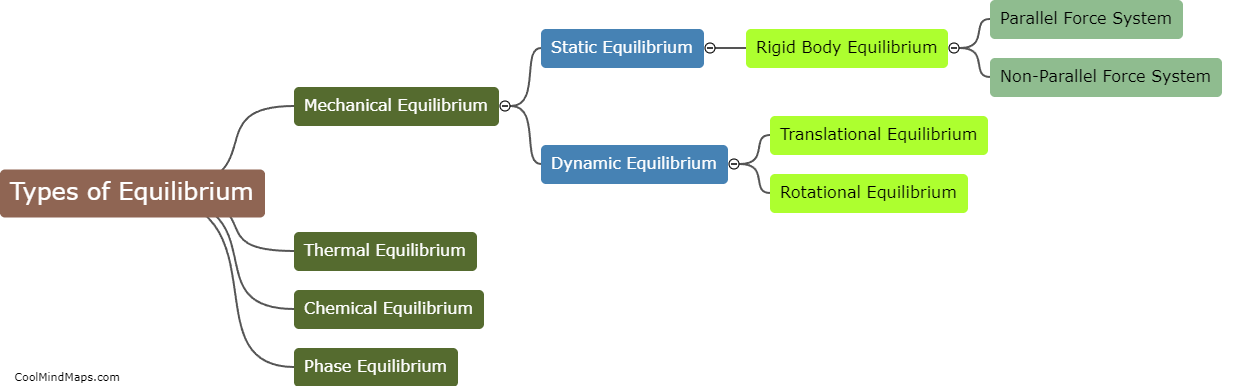
What are the different types of equilibrium in thermodynamics?
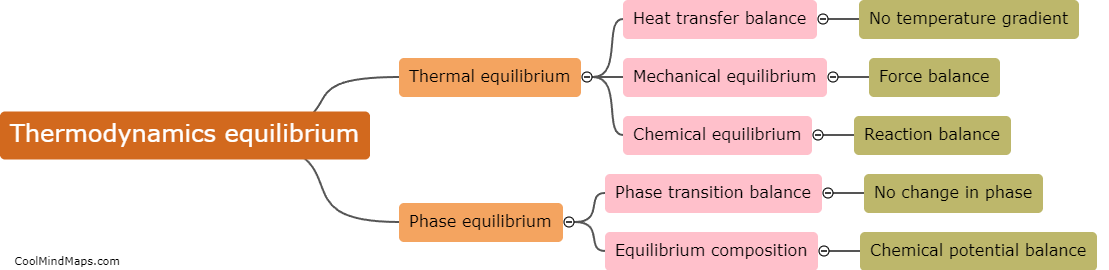
In thermodynamics, there are three main types of equilibrium: thermal equilibrium, mechanical equilibrium, and chemical equilibrium. Thermal equilibrium occurs when two or more systems are at the same temperature and there is no net transfer of heat between them. Mechanical equilibrium is achieved when there is no net force or movement occurring within a system. It involves a balance between the forces exerted on an object and the forces resisting its motion. Finally, chemical equilibrium is a state in which the forward and reverse reactions of a chemical reaction occur at the same rate, resulting in a stable composition of the reaction system. These three types of equilibrium are crucial in understanding and analyzing thermodynamic systems and processes.
This mind map was published on 18 September 2023 and has been viewed 92 times.