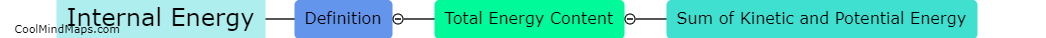What is the relationship between enthalpy and total energy?
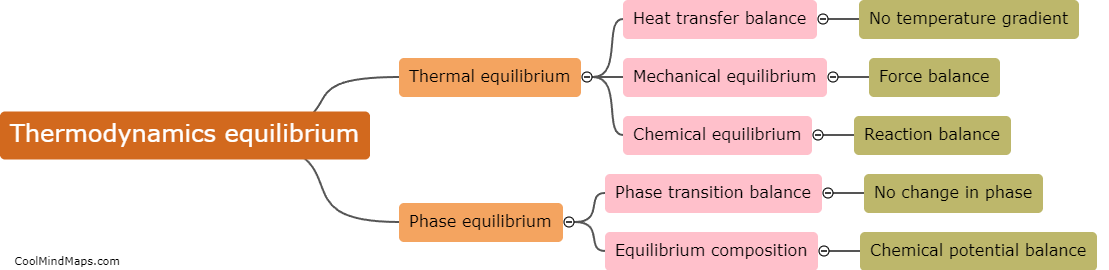
Enthalpy and total energy are closely related concepts in thermodynamics. Total energy refers to the overall energy content of a system, including both its internal energy and any potential or kinetic energy associated with it. Enthalpy, on the other hand, is a thermodynamic property that accounts for the internal energy of a system as well as the work it may do on its surroundings. In simple terms, enthalpy can be thought of as the sum of a system's internal energy and the energy required to change its volume or pressure. Therefore, enthalpy represents the total energy of a system, encompassing both its internal energy and energy exchange at its boundaries.
This mind map was published on 19 September 2023 and has been viewed 109 times.