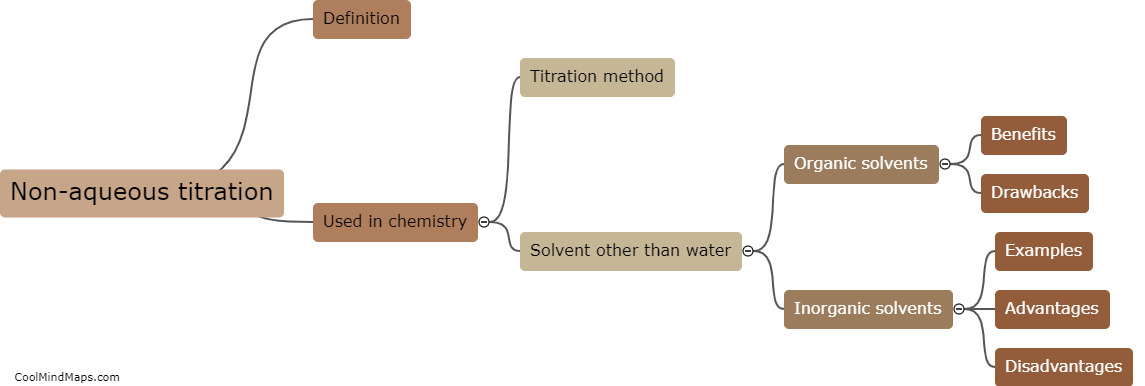
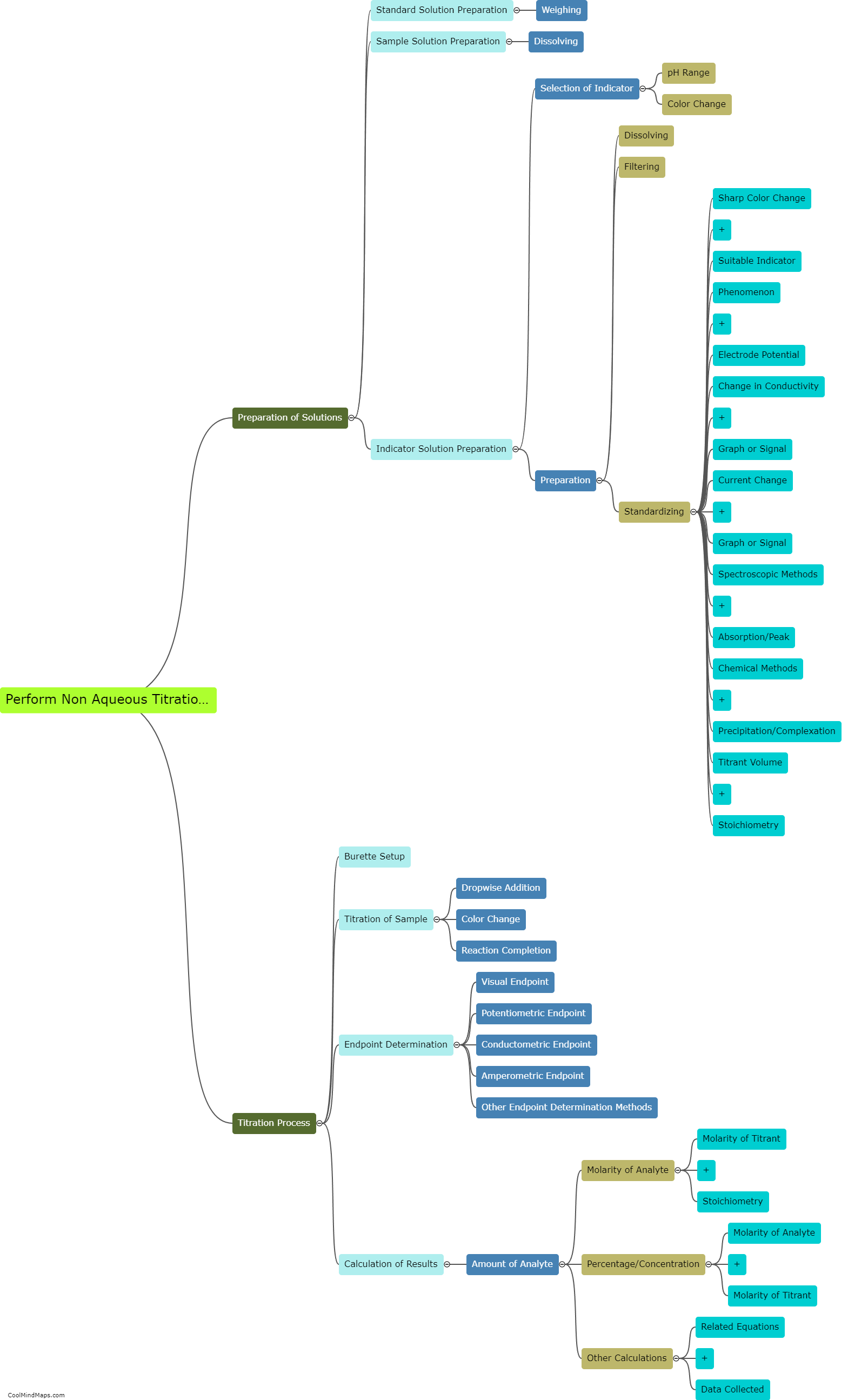
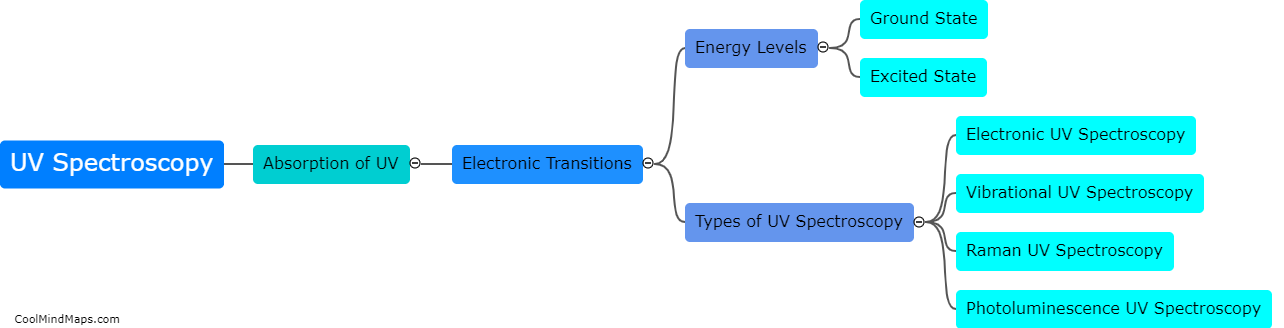
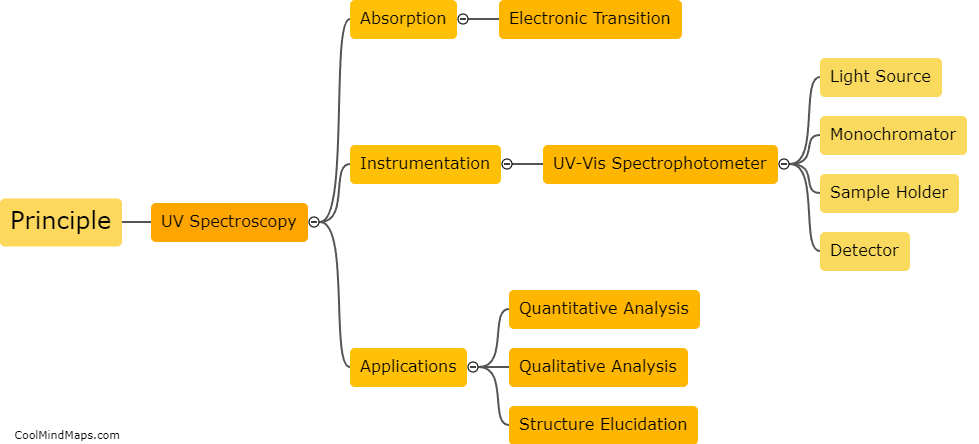
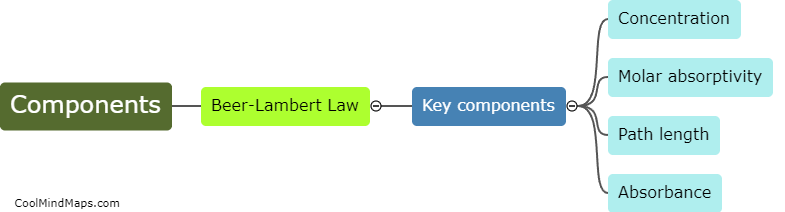
What are the key components of the Beer-Lambert law?
The Beer-Lambert law, also known as the Beer's law or Lambert-Beer's law, describes the relationship between the concentration of a solution and its absorbance of light. The key components of the Beer-Lambert law include the concentration of the absorbing species, the path length through which the light passes, and the molar absorptivity of the absorbing species at a specific wavelength. The law states that the absorbance of a solution is directly proportional to the concentration of the species and the path length, and is also influenced by the molar absorptivity. This law is widely used in spectrophotometry to quantify the concentration of a solute in a solution by measuring the absorbance of light passing through it.
This mind map was published on 8 October 2023 and has been viewed 98 times.