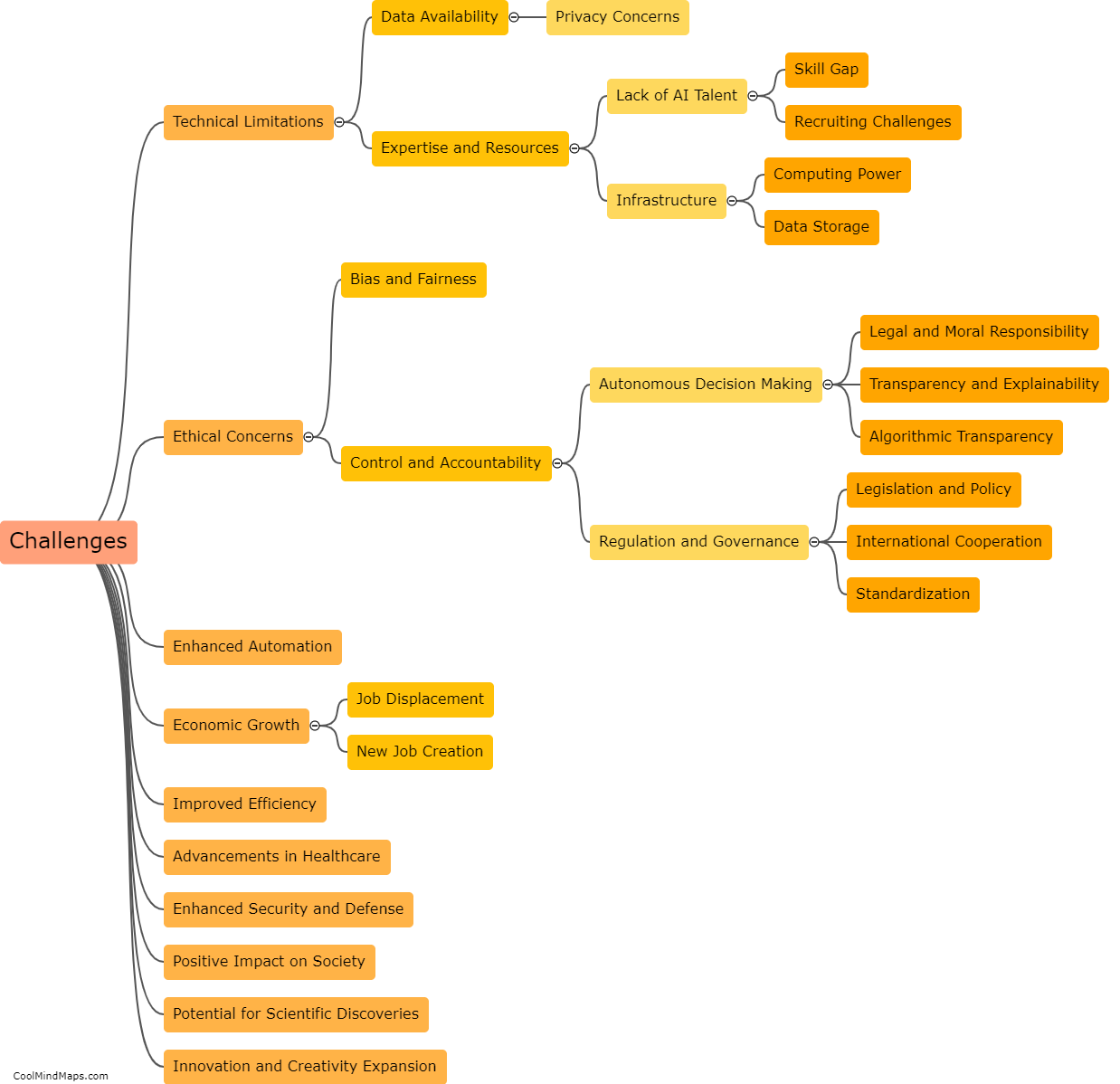
What are the elements in the first transition series?
The first transition series refers to the first 10 elements in the d-block of the periodic table, starting from scandium (Sc) and ending with zinc (Zn). These elements exhibit similar properties due to their similar electron configurations, where the outermost electron is entering or occupying a d orbital. The elements in this series include titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and finally zinc (Zn). They are known for their ability to form multiple oxidation states and their tendency to undergo inner orbital (d-orbital) electron transfers, making them important in various industrial and biological processes.

This mind map was published on 9 October 2023 and has been viewed 91 times.