What are the steps involved in financial planning for clinical research?
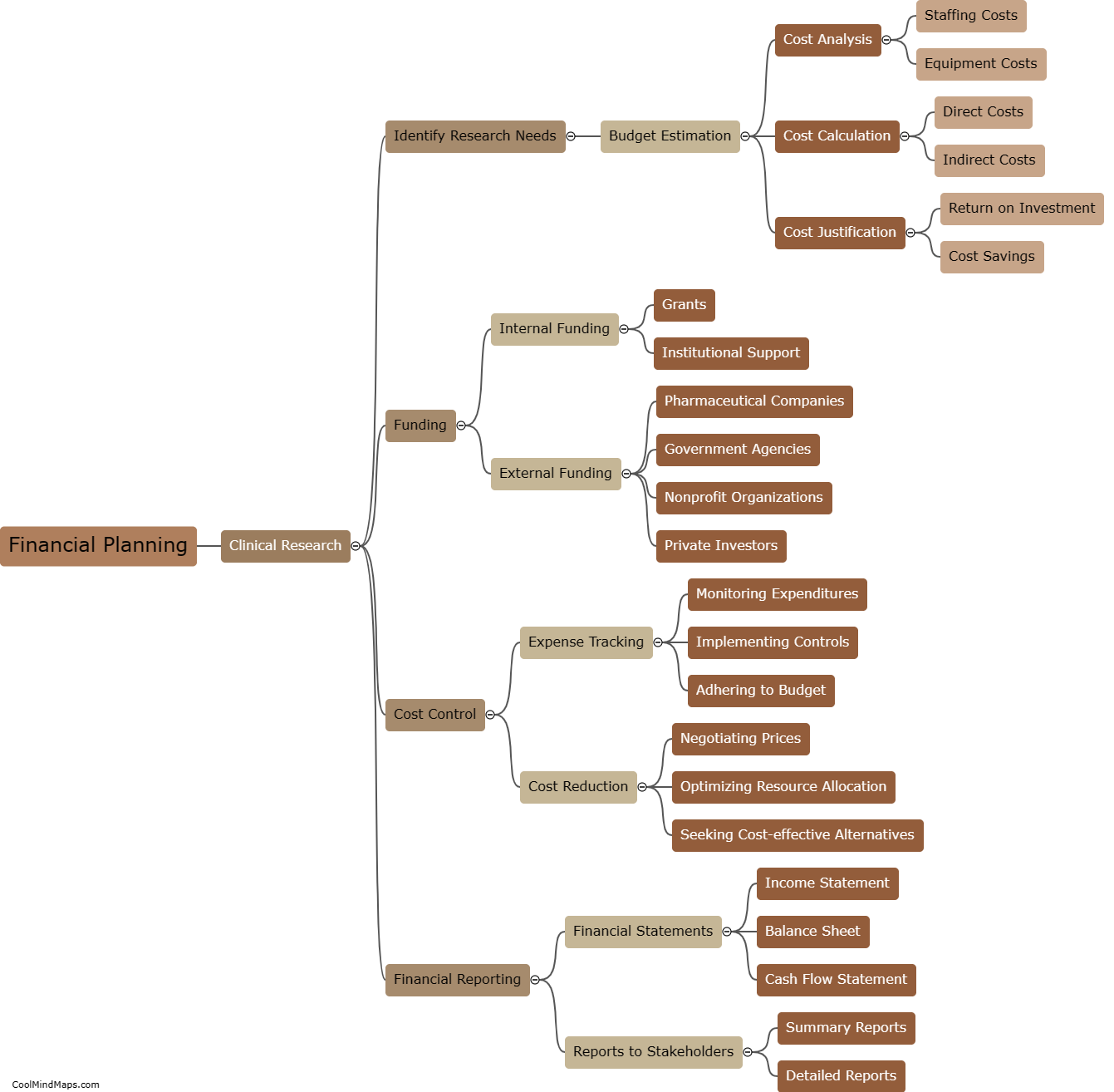
Financial planning for clinical research involves several important steps. Firstly, it is essential to estimate the overall budget required for the research project. This includes costs for personnel, supplies, equipment, and any additional expenses related to the study. Next, potential sources of funding should be identified, such as grants, contracts, or donations. It is crucial to review eligibility criteria and deadlines for each funding source to ensure a successful application. After securing funding, a detailed budget should be created, outlining specific allocations for each research component. Regular monitoring and tracking of expenses against the budget are necessary throughout the project to ensure adherence to financial goals. Additionally, contingency plans should be developed to address unexpected costs or delays. By following these steps, researchers can effectively plan and manage the financial aspects of their clinical research to ensure its successful execution.
This mind map was published on 1 December 2023 and has been viewed 114 times.