What are the 4 types of FDA approval?
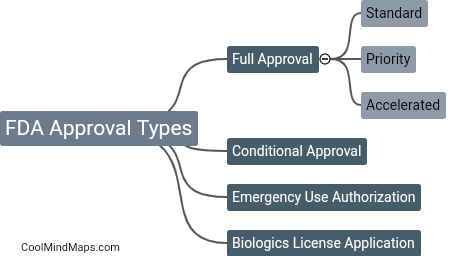
There are four types of FDA approval: premarket approval (PMA), 510(k) clearance, accelerated approval, and expanded access. Pre-market approval is required for high-risk medical devices and involves stringent testing and clinical trials. 510(k) clearance is required for devices that are substantially equivalent to an already approved device, and is based on the device's safety and effectiveness compared to the predicate device. Accelerated approval allows for faster approval of drugs that treat serious or life-threatening conditions and are based on surrogate or intermediate endpoints. Expanded access allows patients with serious or life-threatening conditions to access experimental drugs or devices outside of clinical trials.
This mind map was published on 21 May 2023 and has been viewed 178 times.